Plasma CHI3L1 associates with brain volume loss and glial activation in multiple sclerosis
- PMID: 40379482
- PMCID: PMC12573333
- DOI: 10.1136/jnnp-2025-336063
Plasma CHI3L1 associates with brain volume loss and glial activation in multiple sclerosis
Abstract
Background: Multiple sclerosis (MS) progression independent of relapses is driven by brain innate immune cell activation. The aim of this study was to evaluate the association between chitinase-3-like protein 1 (CHI3L1), expressed in brain by astrocytes and microglia, measured from blood and smouldering inflammation measured using 18 kDa translocator protein (TSPO) positron emission tomography (PET) in patients with MS.
Methods: The study cohort included 55 patients with MS (25 progressive MS (PMS) and 30 relapsing remitting MS (RRMS)) and 17 healthy controls (HC). CHI3L1 was measured with commercial ELISA from plasma samples. A subcohort (44 MS and 9 HC) underwent TSPO-PET to assess [11C]PK11195 distribution volume ratio (DVR) and MRI concurrent to blood sampling. These imaging outcomes were used in respective correlation and linear regression analyses.
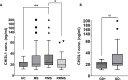
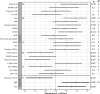
Results: CHI3L1 concentration in plasma was higher in PMS (23.5 ng/mL) compared with HC (16.8 ng/mL, p=0.0055) and RRMS (19.3 ng/mL, p=0.049). CHI3L1 associated with brain [11C]PK11195 DVR in all MS (standardised estimate 0.89, 95% CI 0.23 to 1.55, p=0.010) and in PMS (Spearman correlation ρ=0.58, 95% CI 0.058 to 0.86, p=0.032). Additionally, CHI3L1 was associated with smaller brain volume in both MS (-0.75, -1.38 to -0.11, p=0.023) and PMS (ρ=-0.56, -0.83 to -0.095, p=0.021). Furthermore, CHI3L1 was associated with Expanded Disability Status Scale (0.70, 0.12 to 1.28, p=0.019) and age (0.93, 0.37 to 1.48, p=0.002) among all patients with MS.
Conclusions: Association of CHI3L1 with glial activation and brain volume loss identifies plasma CHI3L1 as a promising biomarker for smouldering inflammation and MS progression-related pathology.
Keywords: MRI; MULTIPLE SCLEROSIS; NEUROIMMUNOLOGY; PET.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
