Tacrolimus dosing in liver transplant recipients using phenotypic personalized medicine: A phase 2 randomized clinical trial
- PMID: 40379675
- PMCID: PMC12084539
- DOI: 10.1038/s41467-025-59739-6
Tacrolimus dosing in liver transplant recipients using phenotypic personalized medicine: A phase 2 randomized clinical trial
Abstract
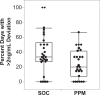
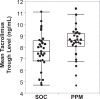
Tacrolimus is the most commonly used immunosuppression drug after solid organ transplantation; however, its dosing is challenging due to substantial inter-individual variability, often resulting in blood levels that deviate from the target therapeutic range. We explored whether a dynamically customized, phenotypic-outcome-guided drug dosing method could improve maintenance of drug trough levels within pre-determined target ranges, focusing on tacrolimus immediately after liver transplantation. This single-center, partially blinded, completed clinical trial involved 62 adults undergoing liver transplantation, block randomized into parallel groups: standard-of-care (SOC) clinician-determined or Phenotypic Personalized Medicine (PPM)-guided tacrolimus dosing. The primary outcome was percentage of post-transplant days with large (>2 ng/mL) deviations from the target range. At trial completion, analysis found statistically significant improvement in the PPM group (n = 27): 24.2% of days showing large deviations compared to 38.4% in the SOC group (n = 29) (difference -14.2%, 95% CI: -26.7 to -1.5 %, P = 0.029) with no increase in adverse events. These results demonstrate that PPM-guided tacrolimus dosing more effectively maintains drug levels within the target range compared to SOC, suggesting a promising approach to improving drug dosing. The trial was registered at ClinicalTrials.gov with the identifier NCT03527238.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: C.M.H. is an inventor on pending and issued patents (International Patent Application Serial No. PCT/US2014/012111 and PCT/US2015/058892). CMH is a co-inventor of the pending patent WO2015017449. CMH and AZ are co-inventors of the issued patent US2019/0121935A1. C.M.H., A.Z., J.K., and M.L. are co-inventors of the pending patent (63/234,124). The remaining authors declare no competing interests.
Figures



References
-
- Zarrinpar, A. & Busuttil, R. W. Liver transplantation: past, present and future. Nat. Rev. Gastroenterol. Hepatol.10, 434–440 (2013). - PubMed
-
- Enderby, C. & Keller, C. A. An overview of immunosuppression in solid organ transplantation. Am. J. Manag Care21, s12–s23 (2015). - PubMed
-
- Zarrinpar, A. & Busuttil, R. W. Immunomodulating options for liver transplant patients. Expert Rev. Clin. Immunol.8, 565–578 (2012). - PubMed
-
- Yu, S. et al. Influence of CYP3A5 gene polymorphisms of donor rather than recipient to tacrolimus individual dose requirement in liver transplantation. Transplantation81, 46–51 (2006). - PubMed

