Experimental study of cerebral edema and expression of VEGF and AQP4 in the penumbra area of rat brain trauma
- PMID: 40379765
- PMCID: PMC12084337
- DOI: 10.1038/s41598-025-02071-2
Experimental study of cerebral edema and expression of VEGF and AQP4 in the penumbra area of rat brain trauma
Abstract
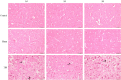
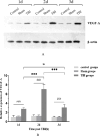
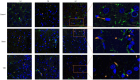
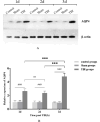
Traumatic brain injury (TBI) has a high disability rate and a high fatality rate. Traumatic penumbra (TP) is a potentially reversible area around the core area of brain trauma with cerebral edema as the main pathological change, which is a breakthrough to improve the prognosis of patients with TBI and reduce the mortality and disability rate of TBI. Unfortunately, the pathophysiological mechanism of TP is still not fully understood. In this study, we established a moderate traumatic brain injury model in rats and detected pathological molecular markers in TP. Protein content of IgG, VEGF, and AQP4 were detect respectively by HE, Immunofluorescence, and western blot. To investigate the time-varying characteristics of TP, to provide a reference for research and development and screening of TBI targeted drugs. Our experiment showed mainly intracellular edema and vascular edema in TP, first intracellular then vascular edema was dominant. IgG, VEGF, and AQP4 in TP increased significantly. On the second day, AQP4 decreased, and third day AQP4 increased again. We found that in the early stage of TBI cerebral edema developed and it is related to the increase of BBB permeability, upregulation of VEGF and AQP4. Suggesting potential targets for treatment of TP.
Keywords: AQP4; BBB; Cerebral edema; Traumatic penumbra; VEGF.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Institutional review board statement: All animal experiments were reviewed and approved by the Institutional Animal Care and Committee of The Seventh People’s Hospital of Chongqing (approval number 479/ March 19,2020). All the experiments were carried out in the army special occupational disease prevention and Control Laboratory of Field Surgery Research Department, Army Characteristic Medical Center, Army Medical University. All experimental animals were treated in accordance with GB/T 35892 − 2018 Guidelines for ethical review of the welfare of experimental animals and guidelines for the management and use of experimental animals (8th edition).
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

