Evaluation of tumor targets selected from public genomic databases for imaging of pancreatic ductal adenocarcinoma
- PMID: 40379788
- PMCID: PMC12084321
- DOI: 10.1038/s41598-025-00517-1
Evaluation of tumor targets selected from public genomic databases for imaging of pancreatic ductal adenocarcinoma
Abstract
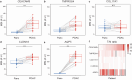
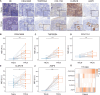
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive malignancy with a 5-year survival rate of approximately 5-7%, and complete surgical resection remains the only curative treatment but is often unfeasible. Fluorescence-guided surgery (FGS) using tumor-targeted probes may improve tumor visualization and facilitate complete resection. This study aimed to identify and validate tumor targets for FGS during PDAC resection procedures. RNA expression data from over 4000 cell surface genes, obtained from public genomic databases, were analyzed to identify genes encoding PDAC-associated proteins. Eleven potential tumor targets were identified, including CEACAM5, TMPRSS4, COL17A1, CLDN18, and AQP5. Protein expression was evaluated by immunohistochemistry (IHC) in tissues from 44 PDAC and 7 chronic pancreatitis (CP) patients. All targets, except COL17A1, showed significantly higher expression in PDAC tissue compared to healthy pancreatic, CP, and duodenal tissue (p < 0.001), as well as in tumor-positive versus tumor-negative lymph nodes. Especially CEACAM5, TMPRSS4, and AQP5 were identified as the most promising targets for distinguishing PDAC from healthy tissues and detecting lymph node metastasis during FGS. The development of probes targeting multiple markers, such as AQP5 with CEACAM5 and/or TMPRSS4, may help overcome interpatient variability and enhance detection across patients.
Keywords: Data-driven approach; Fluorescence-guided surgery; Molecular imaging; Pancreatic cancer; Tumor-target selection.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All procedures involving human participants complied with the ethical standards of the institutional and/or national research committee and followed the principles outlined in the 1964 Helsinki Declaration, including its amendments or equivalent ethical standards. The Ethics Committee of the Leiden University Medical Center reviewed and approved this study (protocol code B20.052, approval date: 17 December 2020). The requirement for informed consent was waived by the Ethics Committee.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

