Real-time peri-operative microcalcification detection in superficial breast tissues
- PMID: 40379809
- PMCID: PMC12084397
- DOI: 10.1038/s41598-025-01629-4
Real-time peri-operative microcalcification detection in superficial breast tissues
Abstract
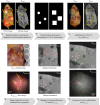
Surgical management of ductal carcinoma in situ (DCIS), particularly in cases involving suspicious morphology and orientation of microcalcifications, remains a primary treatment option. However, the lack of real-time technical assistance in the form of an intraoperative surgical tool for detection of microcalcifications in the resection margins presents a significant challenge. In the context of breast conserving surgery, ex-vivo imaging of excised breast tissues slices from 12 patients was conducted. By employing a cross-polarized multispectral microcamera setup for tissue visualization an imaging depth of up to 2 mm was achieved. The microcamera provides the clinician with a clear color image with magnification allowing features down to 50 μm to be seen on the resection surface. Mammography images were used for accurate cross-correlation, enabling the identification of microcalcifications in the microcamera images. Detection efficacy of microcalcifications in microcamera images was notably influenced by both calcification clustering and distribution depth within the tissue. Calcifications within the 2 mm range were detected through their distinct optical manifestations in relation to the adjacent tissues. Four independent reviewers-two medical and two technical-achieved an average sensitivity of 77.8%, specificity of 80.0%, and overall accuracy of 79.0%. This study demonstrates the potential of an integrated microcamera and cross-polarized setup for non-invasive, real-time detection of microcalcifications in superficial breast tissues. By focusing on the superficial 2 mm, this approach shows promising results and offers substantial opportunities for future research and clinical applications.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
The diagnostic sensitivity of dynamic contrast-enhanced magnetic resonance imaging and breast-specific gamma imaging in women with calcified and non-calcified DCIS.Acta Radiol. 2014 Jul;55(6):668-75. doi: 10.1177/0284185113505086. Epub 2013 Sep 16. Acta Radiol. 2014. PMID: 24043881
-
Local tumor recurrence following breast-conservation therapy: correlation of histopathologic findings with detection method and mammographic findings.Radiology. 1999 Sep;212(3):829-35. doi: 10.1148/radiology.212.3.r99se41829. Radiology. 1999. PMID: 10478253
-
[Radiographic image magnification as quality control of microcalcification imaging within the scope of histopathological control of breast biopsy tissue].Rofo. 2000 Jan;172(1):68-72. doi: 10.1055/s-2000-280. Rofo. 2000. PMID: 10719466 German.
-
The role of specimen radiography in breast-conserving therapy of ductal carcinoma in situ.Breast. 2016 Apr;26:73-9. doi: 10.1016/j.breast.2015.12.014. Epub 2016 Feb 1. Breast. 2016. PMID: 27017245
-
Stereotactic 14 gauge core-biopsy of the breast: results from 101 patients.Aust N Z J Surg. 1996 Sep;66(9):585-91. doi: 10.1111/j.1445-2197.1996.tb00824.x. Aust N Z J Surg. 1996. PMID: 8859155 Review.
References
-
- Eberl, M. M., Fox, C. H., Edge, S. B., Carter, C. A. & Mahoney, M. C. BI-RADS classification for management of abnormal mammograms. J. Am. Board. Fam Med.19 (2), 161–164 (2006). - PubMed
-
- IKNL, R. I. V. M. Monitor bevolkingsonderzoek borstkanker 2018/2019 (september), 1–6. http://www.rivm.nl/monitoring-evaluatie-bevolkingsonderzoek-borstkanker (2020).
-
- Sharma, T., Radosevich, J. A., Pachori, G. & Mandal, C. C. A molecular view of pathological microcalcification in breast cancer. J. Mammary Gland Biol. Neoplasia [Internet]. 21 (1–2), 25–40. 10.1007/s10911-015-9349-9 (2016). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

