Sensitizing solid tumors to CAR-mediated cytotoxicity by lipid nanoparticle delivery of synthetic antigens
- PMID: 40379831
- PMCID: PMC12305469
- DOI: 10.1038/s43018-025-00968-5
Sensitizing solid tumors to CAR-mediated cytotoxicity by lipid nanoparticle delivery of synthetic antigens
Abstract
Chimeric antigen receptor (CAR) T cell immunotherapy relies on CAR targeting of tumor-associated antigens; however, heterogenous antigen expression, interpatient variation and off-tumor expression by healthy cells remain barriers. Here we develop synthetic antigens to sensitize solid tumors for recognition and elimination by CAR T cells. Unlike tumor-associated antigens, we design synthetic antigens that are orthogonal to endogenous proteins to eliminate off-tumor targeting and that have a small genetic footprint to facilitate efficient tumor delivery to tumors by lipid nanoparticles. Using a camelid single-domain antibody (VHH) as a synthetic antigen, we show that adoptive transfer of anti-VHH CAR T cells to female mice bearing VHH-expressing tumors reduced tumor burden in multiple syngeneic and xenograft models of cancer, improved survival, induced epitope spread, protected against tumor rechallenge and mitigated antigen escape in heterogenous tumors. Our work supports the in situ delivery of synthetic antigens to treat antigen-low or antigen-negative tumors with CAR T cells.
© 2025. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests: G.A.K. reports equity or consulting roles for Sunbird Bio, Port Therapeutics, Send Biotherapeutics and Ridge Biotechnologies. The terms of this arrangement were reviewed and approved by Georgia Tech in accordance with its conflict-of-interest policies. L.G., A.H.Z., D.V., P.J.S., C.S.C., H.J.L., C.A.T. and G.A.K. are listed as inventors on a patent application related to the results of this paper, titled ‘Synthetic Antigens as Chimeric Antigen Receptor (CAR) Ligands and Uses Thereof’ (US20230390335A1). The patent applicant is the Georgia Tech Research Corporation.
Figures



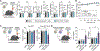

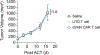
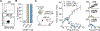

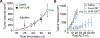







References
MeSH terms
Substances
Grants and funding
- F31-CA271803/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01EB032822/U.S. Department of Health & Human Services | NIH | National Institute of Biomedical Imaging and Bioengineering (NIBIB)
- UL1TR000454/U.S. Department of Health & Human Services | NIH | National Center for Advancing Translational Sciences (NCATS)
- 5T32EB006343, F31-CA271803/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- S10 OD016264/OD/NIH HHS/United States
- 1S10OD016264-01A1/Shurl and Kay Curci Foundation (Shurl & Kay Curci Foundation)
- 5T32GM145735/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- F31 CA271803/CA/NCI NIH HHS/United States
- R01 EB032822/EB/NIBIB NIH HHS/United States
- DP2 HD091793/HD/NICHD NIH HHS/United States
- T32 EB006343/EB/NIBIB NIH HHS/United States
- DP1 CA280832/CA/NCI NIH HHS/United States
- 5T32EB006343/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- DP1-CA280832/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01CA273878/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- DP2-HD091793/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- DGE-1451512/National Science Foundation (NSF)
- UL1 TR000454/TR/NCATS NIH HHS/United States
- R01 CA273878/CA/NCI NIH HHS/United States
- T32 GM145735/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

