The Conceivable Role of Metabolic Syndrome in the Pathogenesis of Alzheimer's Disease: Cellular and Subcellular Alterations in Underpinning a Tale of Two
- PMID: 40379890
- PMCID: PMC12084261
- DOI: 10.1007/s12017-025-08832-6
The Conceivable Role of Metabolic Syndrome in the Pathogenesis of Alzheimer's Disease: Cellular and Subcellular Alterations in Underpinning a Tale of Two
Abstract
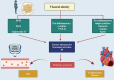
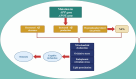
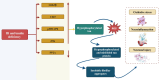
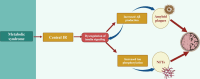
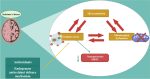
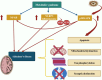
Alzheimer's disease (AD)is an age-related neurodegenerative disease characterized by memory decline and cognitive impairment .AD is common in people aged > 65 years, though most of AD cases are sporadic, which accounts for 95%, and 1-5% of AD is caused by familial causes . The causes of AD are aging, environmental toxins, and cardiometabolic factors that induce the degeneration of cholinergic neurons. It has been shown that the metabolic syndrome which is a clustering of dissimilar constituents including insulin resistance (IR), glucose intolerance, visceral obesity, hypertension, and dyslipidemia is implicated in the pathogenesis of AD. Metabolic syndrome disapprovingly affects cognitive function and the development in AD by inducing the development of oxidative stress, neuroinflammation, and brain IR. These changes, together with brain IR, impair cerebrovascular reactivity causing cognitive impairment and dementia. Nevertheless, the fundamental mechanism by which metabolic syndrome persuades AD risk is not entirely explicated. Accordingly, this review aims to discuss the connotation between metabolic syndrome and AD. In conclusion, metabolic syndrome is regarded as a possible risk factor for the initiation of AD neuropathology by diverse signaling pathways such as brain IR, activation of inflammatory signaling pathways, neuroinflammation, defective proteostasis, and dysregulation of lipid mediators.
Keywords: Alzheimer’s disease; Insulin resistance; Metabolic syndrome; Neuroinflammation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing Interests: The authors declare no competing interests. Ethical Approval: Not applicable. Consent to Participate: Not applicable. Consent for Publication: Not applicable.
Figures
References
-
- Abo El-Magd, N. F., El-Mesery, M., El-Karef, A., & El-Shishtawy, M. M. (2018). Glycyrrhizin ameliorates high fat diet-induced obesity in rats by activating NrF2 pathway. Life Sciences,193, 159–170. 10.1016/j.lfs.2017.11.005 - PubMed
-
- Alexandre-Santos, B., Magliano, D. C., Giori, I. G., Medeiros, G. R. D. O., Vieira, C. P., Conte-Junior, C. A., et al. (2022). Renin-angiotensin system modulation through enalapril and/or exercise training improves visceral adiposity in obese mice. Life Sciences,291, 120269. 10.1016/j.lfs.2021.120269 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

