Impact of adjuvant therapy on outcomes of cancer of the stomach and gastroesophageal junction in the real-world
- PMID: 40379901
- PMCID: PMC12378129
- DOI: 10.1007/s10120-025-01624-8
Impact of adjuvant therapy on outcomes of cancer of the stomach and gastroesophageal junction in the real-world
Abstract
Background: Since the FLOT4 gastric cancer (GC) trial, the use of adjuvant chemotherapy has been perceived as limited and its added value questioned. We wanted to objectify this perception and reassess the value of adjuvant chemotherapy in a real-world setting.
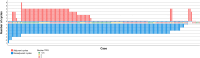
Methods: In our retrospective cohort study we analyzed real-world data from 147 patients with GC or cancer of the gastroesophageal junction (AEG) who received perioperative FLOT. Data originated from clinical care at the university hospital, local hospitals and medical practices. Clinicopathologic data, survival outcomes, and targetable biomarkers were analyzed.
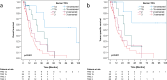
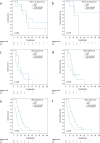
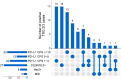
Results: Median overall survival (OS) and tumor specific survival (TSS) were 19.4 ± 2.9 and 19.9 ± 3.1 months, respectively. 84.4% completed all cycles of neoadjuvant chemotherapy. The pathological complete response rate was 11.8%. Adjuvant chemotherapy was initiated in only 42.9%. Survival rates of patients with marked tumor regression (TRG1) were not improved by adjuvant chemotherapy. Conversely, patients with partial histopathologic response (TRG2) showed a marked trend and those with minimal histopathologic response (TRG3) showed a significantly longer survival with any number of adjuvant chemotherapy cycles (OS: 22.3 ± 2.6 months versus 8.7 ± 2.4 months, p = 0.005; TSS: 22.3 ± 4.5 months versus 8.7 ± 2.4 months, p = 0.016). Targetable biomarkers PD-L1, Claudin 18.2, HER2 and microsatellite instability were detected in 53.4%, 26.2%, 7.8%, and 3.9% of the TRG2/3 patient subset, respectively.
Conclusions: In the real-world setting, adjuvant chemotherapy proved to be a critical turning point of the FLOT regimen. It should be sought-even in a reduced form-in patients with TRG2/3.
Keywords: Adjuvant; Biomarkers; Chemotherapy; Esophagogastric junction; Gastric cancer.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: AL received payment or honoraria from Astra Zeneca, Bayer, BMS, Böhringer Ingelheim, Grünenthal, Janssen, Lilly, MSD, Novartis, Roche, Servier and Tesaro. AL participates on the advisory boards of MSD, BMS and Lilly. AL has a leadership role in the Board of Directors European Association of Palliative Care (2023–2026) and in the ESMO Supportive and Palliative Care Faculty (2023–2026). All other authors declare no competing interests. Ethical approval: All procedures followed were in accordance with the ethical standards of the ethics committee [D453/10 (22.10.2010); D525/15 (20.08.2015)] of the University Hospital Schleswig–Holstein, Kiel, Germany, which allowed us to use samples from those patients who had also given written informed consent for prospective scientific use of their patient material (broad consent). The study was performed in compliance with all relevant laws and institutional guidelines and in accordance with the code of ethics of the Helsinki Declaration of 1964 and later versions.
Figures

References
-
- Varon C, Megraud F. Stomach cancer: still one of the main cancer types worldwide. Wild CP, Weiderpass E, Steward BW, editors. Lyon: International Agency for Research on Cancer; 2020. p. 10.
-
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. - PubMed
-
- Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948–57. - PubMed
-
- Becker K, Mueller JD, Schulmacher C, Ott K, Fink U, Busch R, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. 2003;98(7):1521–30. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

