Observational Study of Corticosteroid Phobia Using the TOPICOP Score among Adults and Caregivers of Children with Atopic Dermatitis in Japan
- PMID: 40379942
- PMCID: PMC12126417
- DOI: 10.1007/s13555-025-01439-6
Observational Study of Corticosteroid Phobia Using the TOPICOP Score among Adults and Caregivers of Children with Atopic Dermatitis in Japan
Abstract
Introduction: Topical corticosteroid (TCS) phobia, which tends to interfere with the continuation of TCS treatment for atopic dermatitis (AD), has not been elucidated in Japan using the topical corticosteroid phobia (TOPICOP) scale. We aimed to clarify TCS phobia among patients with AD in Japan and evaluate its relationship with AD conditions.
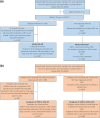
Methods: This observational study, using the database of health receipt (between October 2021 and October 2022) and online questionnaire (conducted in October 2022) data, included adult respondents with and without AD and caregivers who responded to the questionnaire about their children aged ≤ 18 years, with and without AD. The TOPICOP scores measuring TCS phobia were summarized and compared according to AD severity, consultation with a doctor, and presence of AD using an analysis of covariance (ANCOVA) model (covariates: sex and age).
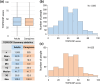
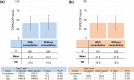
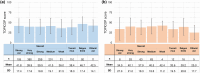
Results: In this study, 1507 adults with AD and 525 caregivers of children with AD were included. Among adults with AD, the mean TOPICOP score (± standard deviation) was 42.5 ± 18.9, while it was 41.7 ± 18.6 among caregivers of children with AD. Of the three TOPICOP domains (knowledge and beliefs, fears, and behaviors), the fear domain questions showed the highest percentage of agreement in both adults and caregivers of children with AD. In the ANCOVA models, the scores did not differ significantly according to AD severity, consultation with a doctor, or the presence of AD in adults and caregivers but significantly differed by sex and age (both p < 0.001) in adults.
Conclusion: We obtained the latest TOPICOP scores that were independent of AD conditions in Japan. Most adults and caregivers of children with AD had TCS phobia, regardless of the AD conditions. Periodic education may be required for a wide range of patients and caregivers to ease TCS phobia and enable them to continue appropriate AD treatment.
Keywords: Atopic dermatitis; Japan; Patient reported outcome measures; Topical corticosteroid phobia; Topical corticosteroid phobia (TOPICOP) scale.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Takeshi Nakahara has received consulting fees and/or speaker honoraria from Mitsubishi Tanabe Pharma, Taiho Pharmaceutical, Torii Pharmaceutical, Maruho, Sanofi, AbbVie, Eli Lilly Japan, Sun Pharma and Otsuka Pharmaceutical. Hiroyuki Murota has received consulting fees and/or speaker honoraria from Maruho Co., Ltd., Sanofi K.K., Pfizer Japan Inc., Torii Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., AbbVie GK, and Otsuka Pharmaceutical Co., Ltd. Shinichi Noto has received speaker honoraria from Otsuka Pharmaceutical. Miyuki Matsukawa, Rikiya Toda, Yasuhito Konishi, and Daisaku Michikami are employees of Otsuka Pharmaceutical Co., Ltd. Ethical Approval: This study was approved on October 21, 2022 by the Research Ethics Committee of Research and Development Division, Otsuka Pharmaceutical Co., Ltd. (approval no: 221013). The dataset used in this study was purchased from the DeSC. We have permission to use this database for the purposes of the research presented in this study. As we used secondary anonymized data, no individual-level informed consent was obtained. The survey was conducted in accordance with the Ethical Guidelines for Medical and Biological Research Involving Human Subjects in Japan and Declaration of Helsinki.
Figures

References
-
- Morita E. Analysis and organization of data on the actual number of patients with atopic dermatitis, causes, and aggravating factors, FY2001 Ministry of Health, Labour and Welfare Research Grant: Research report on immunology/allergy diseases. 2002:184–6.
LinkOut - more resources
Full Text Sources

