High-purity AAV vector production utilizing recombination-dependent minicircle formation and genetic coupling
- PMID: 40379974
- PMCID: PMC12162853
- DOI: 10.1038/s44321-025-00248-w
High-purity AAV vector production utilizing recombination-dependent minicircle formation and genetic coupling
Abstract
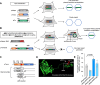
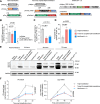
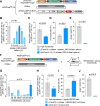
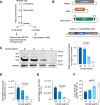
Triple transfection of HEK293 cells is the most widely used method for producing recombinant adeno-associated virus (rAAV), a leading gene delivery vector for human gene therapy. Despite its tremendous success, this approach generates several vector-related impurities that could potentially compromise the safety and potency of rAAV. In this study, we introduce a method for high-purity AAV vector production utilizing recombination-dependent minicircle formation and genetic coupling (AAVPureMfg). Compared with traditional triple transfection, AAVPureMfg substantially improves vector purity by reducing prokaryotic DNA contaminants by 10- to 50-fold and increasing the full capsid ratio up to threefold. Mechanistically, Bxb1-mediated excision of the transgene cassette generates a minicircle cis construct devoid of bacterial sequences and ensures synchronized colocalization of trans and cis constructs in productive cells. Furthermore, we developed iterations that enhance vector genome homogeneity and streamline the production of rAAV with various transgenes, serotypes, and ITR configurations. Overall, our findings demonstrate that AAVPureMfg overcomes the inherent limitations associated with triple transfection, offering a broadly applicable and easy-to-implement method for producing high-purity rAAV with reduced plasmid costs.
Keywords: AAV Manufacturing; AAVPureMfg; Empty Capsid; Plasmid Backbone; Vector Impurities.
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. HL, GG, and DW are inventors of a patent application filed by the University of Massachusetts Chan Medical School concerning the work described in this study. EB was an employee of Pfizer Inc. when the study was conducted. GG is a scientific co-founder of Voyager Therapeutics, Adrenas Therapeutics and Aspa Therapeutics, and holds equity in these companies.
Figures




Similar articles
-
Producing high-quantity and high-quality recombinant adeno-associated virus by low-cis triple transfection.Mol Ther Methods Clin Dev. 2024 Mar 12;32(2):101230. doi: 10.1016/j.omtm.2024.101230. eCollection 2024 Jun 13. Mol Ther Methods Clin Dev. 2024. PMID: 38558570 Free PMC article.
-
Production of Recombinant Adeno-associated Virus Vectors Using Suspension HEK293 Cells and Continuous Harvest of Vector From the Culture Media for GMP FIX and FLT1 Clinical Vector.Mol Ther. 2016 Feb;24(2):287-297. doi: 10.1038/mt.2015.187. Epub 2015 Oct 6. Mol Ther. 2016. PMID: 26437810 Free PMC article.
-
[Recombinant adeno-associated virus vector related impurities].Sheng Wu Gong Cheng Xue Bao. 2011 May;27(5):717-23. Sheng Wu Gong Cheng Xue Bao. 2011. PMID: 21845838 Review. Chinese.
-
Delivering Transgenic DNA Exceeding the Carrying Capacity of AAV Vectors.Methods Mol Biol. 2016;1382:21-39. doi: 10.1007/978-1-4939-3271-9_2. Methods Mol Biol. 2016. PMID: 26611576 Free PMC article.
-
Production of recombinant adeno-associated virus vectors.Hum Gene Ther. 2005 May;16(5):551-7. doi: 10.1089/hum.2005.16.551. Hum Gene Ther. 2005. PMID: 15916480 Review.
References
-
- Chadeuf G, Ciron C, Moullier P, Salvetti A (2005) Evidence for encapsidation of prokaryotic sequences during recombinant adeno-associated virus production and their in vivo persistence after vector delivery. Mol Ther 12:744–753 - PubMed
MeSH terms
Substances
Grants and funding
- UG3 HL147367/HL/NHLBI NIH HHS/United States
- R01 HL097088/HL/NHLBI NIH HHS/United States
- P01 HL131471/HL/NHLBI NIH HHS/United States
- U19AI149646-01/HHS | National Institutes of Health (NIH)
- U19 AI149646/AI/NIAID NIH HHS/United States
- P01 AI100263/AI/NIAID NIH HHS/United States
- P01 HL158506/HL/NHLBI NIH HHS/United States
- R01 AI121135/AI/NIAID NIH HHS/United States
- R01 NS076991/NS/NINDS NIH HHS/United States
- P01HL158506/HHS | National Institutes of Health (NIH)
- R01NS076991-01,P01AI100263-01,P01HL131471-02,35 R01AI121135,UG3HL147367-01,R01HL097088/HHS | National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources

