Visual feedback and motor memory contributions to sustained motor control deficits in autism spectrum disorder across childhood and into adulthood
- PMID: 40380091
- PMCID: PMC12083110
- DOI: 10.1186/s11689-025-09607-7
Visual feedback and motor memory contributions to sustained motor control deficits in autism spectrum disorder across childhood and into adulthood
Abstract
Background: Autistic individuals show deficits in sustained fine motor control which are associated with an over-reliance on visual feedback. Motor memory deficits also have been reported during sustained fine motor control in autism spectrum disorders (ASD). The development of motor memory and visuomotor feedback processes contributing to sustained motor control issues in ASD are not known. The present study aimed to characterize age-related changes in visual feedback and motor memory processes contributing to sustained fine motor control issues in ASD.
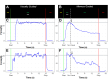
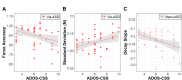
Methods: Fifty-four autistic participants and 31 neurotypical (NT) controls ages 10-25 years completed visually guided and memory guided sustained precision gripping tests by pressing on force sensors with their dominant hand index finger and thumb. For visually guided trials, participants viewed a stationary target bar and a force bar that moved upwards with increased force for 15s. During memory guided trials, the force bar was visible for 3s, after which participants attempted to maintain their force output without visual feedback for another 12s. To assess visual feedback processing, force accuracy, variability (standard deviation), and regularity (sample entropy) were examined. To assess motor memory, force decay latency, slope, and magnitude were examined during epochs without visual feedback.
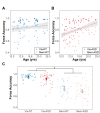
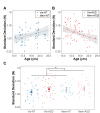
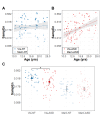
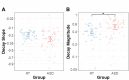
Results: Relative to NT controls, autistic individuals showed a greater magnitude and a trend for a steeper slope of force decay during memory guided trials. Across conditions, the ASD group showed reduced force accuracy (β = 0.41, R2 = 0.043, t79.3=2.36, p = .021) and greater force variability (β=-2.16, R2 = 0.143, t77.1=-4.04, p = .0001) and regularity (β=-0.52, R2 = 0.021, t77.4=-2.21, p = .030) relative to NT controls at younger ages, but these differences normalized by adolescence (age x group interactions). Lower force accuracy and greater force variability during visually guided trials and steeper decay slope during memory guided trials were associated with overall autism severity.
Conclusions: Our findings that autistic individuals show a greater magnitude and tendency for a greater rate of force decay than NT individuals following the removal of visual feedback indicate that motor memory deficits contribute to fine motor control issues in ASD. Findings that sensorimotor differences in ASD were specific to younger ages suggest delayed development across multiple motor control processes.
Keywords: Autism spectrum disorders; Entropy; Fine motor control; Grip force; Motor memory; Sensorimotor; Sensory integration; Visual feedback; Visuomotor.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Adult participants provided written informed consent after a complete description of the study, in accordance with the Declaration of Helsinki. For participants under the age of 18 and adults who were under legal guardianship, a parent or legal guardian provided written informed consent on behalf of the participant, and the participant provided written assent. All study procedures were approved by the University of Kansas Medical Center Institutional Review Board (IRB#: STUDY00140269). Consent for publication: Not applicable. Competing interests: MWM is PI on an investigator initiated clinical trial of behavioral inflexibility in autism funded by Acadia Pharmaceuticals. MWM and ZW received funding from Novartis Pharmaceuticals Corporation for an investigator-initiated study of Phelan McDermid Syndrome. The other authors declare that they have no competing interests.
Figures
Update of
-
Visual feedback and motor memory contributions to sustained motor control deficits in autism spectrum disorder across childhood and into adulthood.Res Sq [Preprint]. 2024 Sep 4:rs.3.rs-4831158. doi: 10.21203/rs.3.rs-4831158/v1. Res Sq. 2024. Update in: J Neurodev Disord. 2025 May 16;17(1):26. doi: 10.1186/s11689-025-09607-7. PMID: 39281871 Free PMC article. Updated. Preprint.
References
-
- Jong MD, Punt M, Groot ED, Minderaa RB, Hadders-Algra M. Minor neurological dysfunction in children with autism spectrum disorder. Dev Med Child Neurol. 2011;53(7):641–6. - PubMed
-
- Hannant P, Cassidy S, Tavassoli T, Mann F. Sensorimotor Difficulties Are Associated with the Severity of Autism Spectrum Conditions. Front Integr Neurosci. 2016;10. Available from: http://journal.frontiersin.org/Article/10.3389/fnint.2016.00028/abstract - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

