Assessing the clinical outcomes of a novel EDOF intraocular lens: a functional classification approach
- PMID: 40380189
- PMCID: PMC12082911
- DOI: 10.1186/s12886-025-04114-8
Assessing the clinical outcomes of a novel EDOF intraocular lens: a functional classification approach
Abstract
Background: Functional assessment can help identify the true extended depth of focus intraocular lenses (EDOF IOLs) on the market. This study aimed to demonstrate the eligibility of the 877PEY ELON IOL (Medicontur Medical Engineering) as a suitable model for this category and to assess its efficacy in clinical settings.
Methods: In total, 38 patients (76 eyes) were enrolled in the study with bilateral implantation of the investigational IOL. For functional classification, a distance-corrected monocular defocus curve was taken 3 months postoperatively. At the 3- and 12-month follow-ups, manifest refraction, monocular and binocular distance, intermediate and near visual acuities, contrast sensitivity, and patient-reported outcomes were recorded.
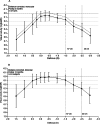
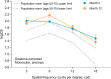
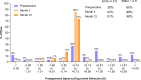
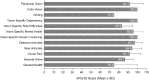
Results: The defocus range (visual acuity [VA] ≤ 0.2 logMAR) on the distance-corrected monocular normalized defocus curve taken at 3 months was 1.7 D, which falls into the Partial Range of Field Extended (later referred to as PRoF-Ex) category, confirming expectations. The binocular depth of focus (VA ≤ 0.1 logMAR) spanned approximately 0.50 D to -1.50 D, and the functional visual acuity (VA ≤ 0.3 logMAR) spanned approximately 1.00 D to -2.50 D. Monocular CSV-1000 outcomes were above the population's normal ranges. 90.9% of the patients were within ± 0.50 D, and 97.7% were within ± 1.00 D SEQ at the 3-month follow-ups. The outcomes of the VFQ-25 questionnaire demonstrated high scores, and the level of spectacle independence, similar to visual acuity, reflected a strong efficacy in distance and intermediate correction with functional near vision. In terms of photopic phenomena, 90% and 87.5% of patients experienced no-to-moderate rates of glare and halos, respectively. The posterior capsular opacification (PCO) rate was 7.89% at the 12-month follow-up. No adverse events were considered serious.
Conclusions: The 877PEY model demonstrated capability as a PRoF-Ex IOL with remarkable performance. It is safe to use and delivers a high degree of patient satisfaction.
Keywords: Cataract surgery; EDOF; Extended depth of focus; Functional classification; Intraocular lens; PRoF-Ex; Presbyopia.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was conducted according to the tenets of the Declaration of Helsinki and was approved by the Health Science and Research Ethical Board Committee of Hungary (OGYÉI, Hungarian Health Authority; ref: OGYEI/34155-6/2020; July 2020). Informed consent was obtained from each patient, and they were free to withdraw from the clinical investigation at any time without giving a reason. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Kondylis G, Klavdianou O, Palioura S. Multifocal and extended depth of focus intraocular lenses. Annals Eye Sci. 2019;4:5. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources

