Antifilarial treatment strategies: a systematic review and network meta-analysis
- PMID: 40380307
- PMCID: PMC12084920
- DOI: 10.1186/s12879-025-11105-z
Antifilarial treatment strategies: a systematic review and network meta-analysis
Abstract
Background: The World Health Organization (WHO) prescribes mass drug administration (MDA) to eradicate lymphatic filariasis within endemic populations. The WHO endorsed using ivermectin with diethylcarbamazine and albendazole (IDA) for MDA in specific settings devoid of onchocerciasis or loiasis. Still, the utilization of IDA in sub-Saharan Africa is restricted due to the potential of diethylcarbamazine to induce severe adverse ocular events in individuals with onchocerciasis.
Aim: We aim to investigate all documented combinations of antifilarial drugs available in the literature using a network meta-analysis (NWM) design, focusing specifically on the treatment of Lymphatic Filariasis (LF).
Methods: A meticulous search was conducted across four electronic databases to identify pertinent studies. Subsequently, a frequentist NWM was executed. Risk ratios (RRs) served as the effect size metric for categorical outcomes, each with a 95% confidence interval (CI).
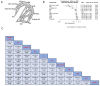
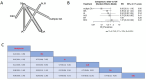
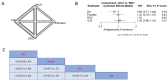
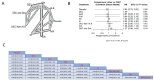
Results: Our study encompassed 45 studies, including 61,369 patients. At six months, multiple doses of diethylcarbamazine plus albendazole (multiple DA) regimens demonstrated superior efficacy in reducing microfilaremia compared to a single intake of DA, diethylcarbamazine, ivermectin, and albendazole with RR and CI as follows: 0.37 [0.19; 0.72], 0.35 [0.17; 0.69], 0.30 [0.14; 0.64], and 0.28 [0.13; 0.57]. The combination of ivermectin plus albendazole (IA) also showed significant efficacy against the use of each of these drugs alone, with RR: 0.74 [0.57; 0.96] for ivermectin and 0.69 [0.53; 0.89] for albendazole, while diethylcarbamazine combined with albendazole showed substantial superiority over albendazole alone or placebo: RR = 0.09 [0.02; 0.36] and 0.08 [0.02; 0.34], respectively. By the twelfth month, diethylcarbamazine, followed by albendazole, ranked superior to IDA and DA: 0.12 [0.02; 0.89] and 0.11 [0.01; 0.79], respectively. At 24 months, no significant differences were found among the assessed drugs in reducing microfilaremia. The comparisons revealed no significant differences between the drug combinations we studied regarding safety and adverse events.
Conclusion: Multiple doses of the DA regimen showed superior efficacy in reducing microfilaremia compared to combinations involving IA, diethylcarbamazine, ivermectin, and albendazole at six and twelve months. However, by the twenty-four-month, no significant differences were found. Safety profiles among interventions were generally comparable, with no specific drug showing superiority in adverse events.
Keywords: Antifilarial drugs; Drug efficacy; Microfilaremia; Network Meta-Analysis; sub-Saharan Africa.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests. Clinical trial number: Not applicable.
Figures


Similar articles
-
Identifying co-endemic areas for major filarial infections in sub-Saharan Africa: seeking synergies and preventing severe adverse events during mass drug administration campaigns.Parasit Vectors. 2018 Jan 31;11(1):70. doi: 10.1186/s13071-018-2655-5. Parasit Vectors. 2018. PMID: 29382363 Free PMC article.
-
An open label, block randomized, community study of the safety and efficacy of co-administered ivermectin, diethylcarbamazine plus albendazole vs. diethylcarbamazine plus albendazole for lymphatic filariasis in India.PLoS Negl Trop Dis. 2021 Feb 16;15(2):e0009069. doi: 10.1371/journal.pntd.0009069. eCollection 2021 Feb. PLoS Negl Trop Dis. 2021. PMID: 33591979 Free PMC article. Clinical Trial.
-
An open label, randomized clinical trial to compare the tolerability and efficacy of ivermectin plus diethylcarbamazine and albendazole vs. diethylcarbamazine plus albendazole for treatment of brugian filariasis in Indonesia.PLoS Negl Trop Dis. 2021 Mar 29;15(3):e0009294. doi: 10.1371/journal.pntd.0009294. eCollection 2021 Mar. PLoS Negl Trop Dis. 2021. PMID: 33780481 Free PMC article. Clinical Trial.
-
Albendazole alone or in combination with microfilaricidal drugs for lymphatic filariasis.Cochrane Database Syst Rev. 2019 Jan 8;1(1):CD003753. doi: 10.1002/14651858.CD003753.pub4. Cochrane Database Syst Rev. 2019. PMID: 30620051 Free PMC article.
-
An analysis of the safety of the single dose, two drug regimens used in programmes to eliminate lymphatic filariasis.Parasitology. 2000;121 Suppl:S147-60. doi: 10.1017/s0031182000007423. Parasitology. 2000. PMID: 11386686 Review.
References
-
- World Health Organization. Global programme to eliminate lymphatic filariasis: progress report 2020.
-
- Thomsen EK, Sanuku N, Baea M, Satofan S, Maki E, Lombore B, et al. Efficacy, safety, and pharmacokinetics of coadministered diethylcarbamazine, Albendazole, and Ivermectin for treatment of Bancroftian filariasis. Clin Infect Dis. 2016;62:334–41. 10.1093/cid/civ882. - PubMed
-
- Tavul L, Laman M, Howard C, Kotty B, Samuel A, Bjerum C, et al. Safety and efficacy of mass drug administration with a single-dose triple-drug regimen of albendazole + diethylcarbamazine + ivermectin for lymphatic filariasis in Papua new Guinea: an open-label, cluster-randomised trial. PLoS Negl Trop Dis. 2022;16:e0010096. 10.1371/journal.pntd.0010096. - PMC - PubMed
-
- Bjerum CM, Ouattara AF, Aboulaye M, Kouadio O, Marius VK, Andersen BJ, et al. Efficacy and safety of a single dose of Ivermectin, diethylcarbamazine, and albendazole for treatment of lymphatic filariasis in Côte D’Ivoire: an Open-label randomized controlled trial. Clin Infect Dis. 2020;71:e68–75. 10.1093/cid/ciz1050. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

