The biological activity and potential of probiotics-derived extracellular vesicles as postbiotics in modulating microbiota-host communication
- PMID: 40380331
- PMCID: PMC12082936
- DOI: 10.1186/s12951-025-03435-6
The biological activity and potential of probiotics-derived extracellular vesicles as postbiotics in modulating microbiota-host communication
Abstract
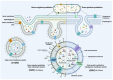
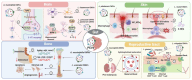
Probiotics such as Lactobacillus and Bifidobacterium spp. have been shown to be critical for maintaining host homeostasis. In recent years, key compounds of postbiotics derived from probiotic metabolism and cellular secretion have been identified for their role in maintaining organ immunity and regulating intestinal inflammation. In particular, probiotic-derived extracellular vesicles (PEVs) can act as postbiotics, maintaining almost the same functional activity as probiotics. They also have strong biocompatibility and loading capacity to carry exogenous or parental active molecules to reach distal organs to play their roles. This provides a new direction for understanding the intrinsic microbiota-host communication mechanism. However, most current studies on PEVs are limited to their functional effects/benefits, and their specific physicochemical properties, composition, intrinsic mechanisms for maintaining host homeostasis, and possible threats remain to be explored. Here, we review and summarize the unique physicochemical properties of PEVs and their bioactivities and mechanisms in mediating microbiota-host communication, and elucidate the limitations of the current research on PEVs and their potential application as postbiotics.
Keywords: Extracellular vesicles (EVs); Host homeostasis; Microbiota-host communication; Postbiotics; Probiotic-derived extracellular vesicles (PEVs).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Yilmaz B, Macpherson AJ. Delving the depths of ‘terra incognita’ in the human intestine - the small intestinal microbiota. Nat Rev Gastroenterol Hepatol. 2024. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

