Conditional progression-free survival in patients with metastatic hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer treated with first-line ribociclib and endocrine therapy: real-world data from the RIBANNA study
- PMID: 40381382
- PMCID: PMC12146538
- DOI: 10.1016/j.esmoop.2025.105105
Conditional progression-free survival in patients with metastatic hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer treated with first-line ribociclib and endocrine therapy: real-world data from the RIBANNA study
Abstract
Background: Progression-free survival (PFS) for patients with metastatic hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) breast cancer significantly improved with cyclin-dependent kinase 4/6 inhibitors as part of first-line treatment. No data is available for these patients on how the risk of progression evolves. Therefore, we analyzed conditional PFS (cPFS), which reflects patient prognosis after initial management, that is, the probability of remaining free from progression in those who have already survived without progression for a given period.
Patients and methods: We analyzed PFS and cPFS for patients free from progression after 12, 24, and 36 months (reference time points) treated with ribociclib and endocrine therapy (ET) as first-line treatment for advanced HR+, HER2- breast cancer (aBC) within the RIBANNA noninterventional study (NCT06311383). Relevant subgroups with established prognostic factors were additionally examined.
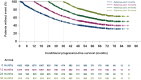
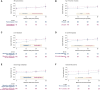
Results: Compared with the median PFS of 35 months (95% confidence interval 32.3-38.4 months) in the overall population, the median cPFS was higher for all reference points: cPFS of 40.5 months (95% confidence interval 35.0-45.5 months) for patients who were progression-free 12 months, cPFS of 53.6 months (95% confidence interval 42.7-not reached months) for 24 months reference point, whereas for the 36 months reference point, the median cPFS was not reached. After patients had reached 2-year disease control, the initial presence of liver metastases or grade 3 disease no longer qualified as poor prognostic factors; internal organ metastases (central nervous system, liver, and lungs) showed a diminishing prognostic impact over time. A short treatment-free interval remained a relevant prognostic factor.
Conclusion: For the first time, increasing cPFS was demonstrated in patients treated with ribociclib and ET. Such information is highly relevant and reassuring for patients with HR+, HER2- aBC, and could be used to aid patient counseling and treatment decision-making, including possible de-escalation strategies. It is also a starting point for identifying dynamic prognostic factors related to long-term survival.
Keywords: RIBANNA; advanced breast cancer; conditional progression-free survival; conditional survival; metastatic breast cancer; ribociclib.
Copyright © 2025 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Robert Koch Institute Zentrum für Krebsregisterdaten: breast cancer 2022. https://www.krebsdaten.de/Krebs/EN/Content/Cancer_sites/Breast_cancer/br... Available at.
-
- European Cancer Information System (ECIS) Breast cancer factsheet in EU-27 countries for. 2022. https://ecis.jrc.ec.europa.eu/sites/default/files/2024-01/jrc_Breast_can... Available at.
-
- Loibl S., Poortmans P., Morrow M., Denkert C., Curigliano G. Breast cancer. Lancet. 2021;397(10286):1750–1769. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

