Glucosylated cholesterol accumulates in atherosclerotic lesions and impacts macrophage immune response
- PMID: 40381699
- PMCID: PMC12197965
- DOI: 10.1016/j.jlr.2025.100825
Glucosylated cholesterol accumulates in atherosclerotic lesions and impacts macrophage immune response
Abstract
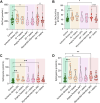
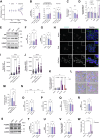
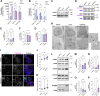
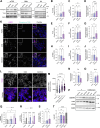
Atherosclerosis can be described as a local acquired lysosomal storage disorder (LSD), resulting from the build-up of undegraded material in lysosomes. Atherosclerotic foam cells accumulate cholesterol (Chol) and glycosphingolipids (GSLs) within lysosomes. This constitutes the ideal milieu for the formation of a side product of lysosomal storage: glucosylated cholesterol (GlcChol), previously found in several LSDs. Using LC-MS/MS, we demonstrated that GlcChol is abundant in atherosclerotic lesions. Patients suffering from cardiovascular diseases presented unaltered plasma GlcChol levels but slightly elevated GlcChol/Chol ratios. Furthermore, we mimicked GlcChol formation in vitro by exposing macrophages (Mφ) to a pro-atherogenic oxidized cholesteryl ester, an atherosclerosis foam cell model. Additionally, Mφ exposed to GlcChol exhibited an enlarged and multinucleated phenotype. These Mφ present signs of decreased proliferation and reduced pro-inflammatory capacity. Mechanistically, the process seems to be associated with activating the AMPK signaling pathway and the cyclin-dependent kinase inhibitor 1 (CDKN1A/p21), in response to DNA damage inflicted by reactive oxygen species (ROS). At the organelle level, exposure to GlcChol impacted the lysosomal compartment, resulting in the activation of the mTOR signaling pathway and lysosomal biogenesis mediated by the transcription factor EB (TFEB). This suggests that high concentrations of GlcChol impact cellular homeostasis. In contrast, under this threshold, GlcChol formation most likely represents a relatively innocuous compensatory mechanism to cope with Chol and GSL build-up within lesions. Our findings demonstrate that glycosidase-mediated lipid modifications may play a role in the etiology of genetic and acquired LSDs, warranting further investigation.
Keywords: glucosylated cholesterol; glycosphingolipids; lysosome; macrophage; multinucleated cells.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interests The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Roth G.A., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. - PMC - PubMed
-
- Alves L.S., Marques A.R.A., Padrão N., Carvalho F.A., Ramalho J., Lopes C.S., et al. Cholesteryl hemiazelate causes lysosome dysfunction impacting vascular smooth muscle cell homeostasis. J. Cell Sci. 2022;135 - PubMed
-
- Domingues N., Marques A.R.A., Calado R.D.A., Ferreira I.S., Ramos C., Ramalho J., et al. Oxidized cholesteryl ester induces exocytosis of dysfunctional lysosomes in lipidotic macrophages. Traffic. 2023;24:284–307. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

