The Effects of Aspartame on Glucose, Insulin, and Appetite-Regulating Hormone Responses in Humans: Systematic Review and Meta-Analyses
- PMID: 40381807
- PMCID: PMC12205327
- DOI: 10.1016/j.advnut.2025.100449
The Effects of Aspartame on Glucose, Insulin, and Appetite-Regulating Hormone Responses in Humans: Systematic Review and Meta-Analyses
Abstract
Aspartame (L-α-aspartyl-L-phenylalanine methyl ester) has been implicated in increased risk of several chronic health conditions, yet underlying mechanisms remain unclear. The objective of this work was to systematically identify and summarize all controlled intervention studies investigating the effects of aspartame consumption on glucose, insulin, and appetite-related hormone responses. Five academic databases, 4 trial registries, and additional resources were searched until June 2024. Search hits were screened, in duplicate, for intervention studies of aspartame compared with comparator, which assessed glucose, insulin, and/or any other appetite-regulating hormone. Results were tabulated, and meta-analyses run where ≥10 studies with similar methodology were found. Risk of bias (RoB) was assessed using RoB-2. Certainty of the evidence was assessed using Grading of Recommendations Assessment, Development, and Evaluation. One hundred one articles were identified, detailing 100 experiments: 79 acute (≤1 d), 8 medium term (2-30 d), and 13 long term (>30 d). Experiments involved healthy adults, individuals with aspartame sensitivity, and individuals with compromised glucose metabolism, varied widely in aspartame provision and comparator/s, and although almost all assessed glucose and/or insulin responses, few experiments investigated other appetite-regulating hormones. Meta-analyses (acute cross-over studies) revealed few effects of aspartame on blood glucose/insulin compared with vehicle or low-calorie sweeteners (LCS), and lower blood glucose/insulin concentrations compared with sugars, other carbohydrates, or other nutritive elements. Over the medium term and longterm, few effects of aspartame were found, and high heterogeneity between studies remained. Similar effects were found in other populations, and other outcomes, with few adverse events. RoB assessments suggested "some concerns" for the majority of studies. The certainty of the evidence for all outcomes in all populations was judged to be "very low." Our findings suggest little to no effects of aspartame consumption on glucose metabolism over the short term or the long term. Further studies over the long term, assessing a range of appetite-regulating hormones and comparing aspartame with other LCS, would be of value. This study was registered in PROSPERO as CRD42024540781 on April 29, 2024.
Keywords: E951; adverse events; appetite; appetite-regulating hormones; aspartame; energy intake; glucose; insulin; low-calorie sweeteners; nonnutritive sweeteners.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest For work in the area of sweet taste and low-calorie sweeteners, in the past 3 y, KMA has previously received research funding from the International Sweeteners Association, BE. She has current funding from a consortium of the American Beverage Association, Arla Foods, Cargill R&D Centre Europe BVBA, DSM-Firmenich SA, International Sweeteners Association, SinoSweet Co., Ltd, Cosun Nutrition Center and Unilever Foods Innovation Centre Wageningen, and from The Coca Cola Company, United States. She has received speaker’s expenses from the International Sweeteners Association, BE; the CBC group, Israel, and EatWell Global. All other authors have no conflicts to declare.
Figures
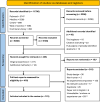
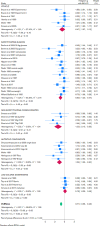
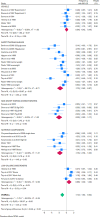
References
-
- World Health Organization Guideline: sugars intake for adults and children. 2020. WHO: Geneva [Internet]https://www.who.int/en/news-room/fact-sheets/detail/healthy-diet [November 30, 2024; date cited]. Available from. - PubMed
-
- Soft drinks industry levy: detailed information. His Majesty’s Revenue & Customs; London: 2018. https://www.gov.uk/topic/business-tax/soft-drinks-industry-levy [November 30, 2024; date cited]. Available from:
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

