Matrix metalloproteinase-10 promotes kidney fibrosis by transactivating β-catenin signaling
- PMID: 40382334
- PMCID: PMC12085647
- DOI: 10.1038/s41420-025-02521-w
Matrix metalloproteinase-10 promotes kidney fibrosis by transactivating β-catenin signaling
Abstract
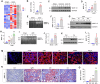
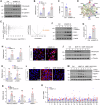
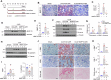
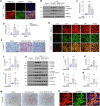
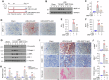
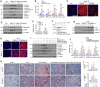
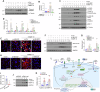
Kidney fibrosis is characterized by excessive accumulation of extracellular matrix (ECM) and serves as a hallmark of chronic kidney disease (CKD). The turnover of ECM is controlled by a family of matrix metalloproteinases (MMPs), endopeptidases that play a crucial role in ECM remodeling and other cellular processes. In this study, we demonstrate that MMP-10 was upregulated in a variety of animal models of kidney fibrosis and human kidney biopsies from CKD patients. Bioinformatics analyses and experimental validation reveal that MMP-10 activated β-catenin in a Wnt-independent fashion. Knockdown of endogenous MMP-10 expression in vivo inhibited β-catenin activation and ameliorated kidney injury and fibrotic lesions, whereas over-expression of exogenous MMP-10 aggravated β-catenin activation and kidney fibrosis after injury. We found that MMP-10 cleaved and activated heparin-binding EGF-like growth factor (HB-EGF) via ectodomain shedding, leading to EGF receptor (EGFR) tyrosine phosphorylation and β-catenin transactivation via a cascade of events involving extracellular signal-regulated kinases and glycogen synthase kinase-3β. Consistently, treatment with erlotinib, a small-molecule EGFR inhibitor, effectively mitigated MMP-10-mediated kidney injury and fibrotic lesions in a dose-dependent fashion. Furthermore, β-catenin activation reciprocally upregulated the expression of MMP-10, thereby perpetuating kidney damage by forming a vicious cycle. Collectively, these results underscore that MMP-10 promotes kidney fibrosis through EGFR-mediated transactivating β-catenin in a Wnt-independent fashion. Our findings suggest that targeting MMP-10 could be a novel strategy for treatment of fibrotic CKD.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: This study was performed in accordance with the Declaration of Helsinki. The animal studies were conducted according to NIH Guide for the Care and Use of Laboratory Animals and approved by the Ethics Committee for Animal Studies at the Nanfang Hospital (NFYY-2020-0953). The use of human kidney specimens in this study was approved by the Institutional Ethics Committee at Nanfang Hospital, with written informed consent from the patients (EFEC-2021-051). To ensure participant anonymity, all personal identifiers were removed from clinical data, and specimens were coded using non-traceable alphanumeric identifiers.
Figures

Similar articles
-
Matrix Metalloproteinase-7 Is a Urinary Biomarker and Pathogenic Mediator of Kidney Fibrosis.J Am Soc Nephrol. 2017 Feb;28(2):598-611. doi: 10.1681/ASN.2016030354. Epub 2016 Sep 13. J Am Soc Nephrol. 2017. PMID: 27624489 Free PMC article.
-
Insulin-like growth factor 2 mRNA-binding protein 3 promotes kidney injury by regulating β-catenin signaling.JCI Insight. 2023 Jan 24;8(2):e162060. doi: 10.1172/jci.insight.162060. JCI Insight. 2023. PMID: 36520532 Free PMC article.
-
Matrix metalloproteinase-10 protects against acute kidney injury by augmenting epidermal growth factor receptor signaling.Cell Death Dis. 2021 Jan 12;12(1):70. doi: 10.1038/s41419-020-03301-3. Cell Death Dis. 2021. PMID: 33436543 Free PMC article.
-
New insights into the role and mechanism of Wnt/β-catenin signalling in kidney fibrosis.Nephrology (Carlton). 2018 Oct;23 Suppl 4:38-43. doi: 10.1111/nep.13472. Nephrology (Carlton). 2018. PMID: 30298654 Review.
-
Wnt/β-catenin signaling and kidney fibrosis.Kidney Int Suppl (2011). 2014 Nov;4(1):84-90. doi: 10.1038/kisup.2014.16. Kidney Int Suppl (2011). 2014. PMID: 26312156 Free PMC article. Review.
Cited by
-
Fibroblast activation and heterogeneity in fibrotic disease.Nat Rev Nephrol. 2025 Sep;21(9):613-632. doi: 10.1038/s41581-025-00969-8. Epub 2025 Jun 19. Nat Rev Nephrol. 2025. PMID: 40537561 Review.
References
-
- Glassock RJ, Warnock DG, Delanaye P. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrol. 2017;13:104–14. - PubMed
-
- Jager KJ, Kovesdy C, Langham R, Rosenberg M, Jha V, Zoccali C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019;96:1048–50. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

