In silico screening of naturally derived dietary compounds as potential butyrylcholinesterase inhibitors for Alzheimer's disease treatment
- PMID: 40382441
- PMCID: PMC12085675
- DOI: 10.1038/s41598-025-98092-y
In silico screening of naturally derived dietary compounds as potential butyrylcholinesterase inhibitors for Alzheimer's disease treatment
Abstract
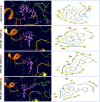
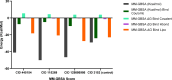
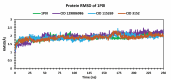
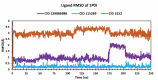
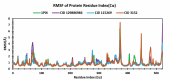
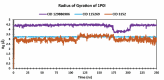
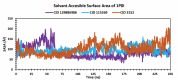
Alzheimer's disease (AD) is a progressive neurodegenerative condition that causes a substantial decline in cognitive functions and affects memory, thinking abilities, and daily behavior. The most prominent hallmark of AD pathogenesis is the formation of amyloid-β plaques, among other associated pathways such as neurofibrillary tangles, mitochondrial dysfunction, neuroinflammation, and oxidative stress. Butyrylcholinesterase (BuChE), an acetylcholine-degrading enzyme, plays a critical role in the progression of Alzheimer's disease, particularly through its involvement in amyloid-β plaque formation. Thus, the inhibition of BuChE is considered a valuable therapeutic strategy for the management of AD. The present study aimed to identify potential bioactive chemicals from naturally occurring dietary compounds that could improve neurocognitive function and appear as a viable treatment for AD by inhibiting the function of BuChE. A small library of 44 natural dietary chemicals from a variety of dietary plants was subjected to comprehensive in silico studies, including molecular docking, molecular mechanics generalized born surface area (MM-GBSA) calculations, pharmacokinetics assessments, toxicity profiles, molecular dynamics (MD) simulation, and density functional theory (DFT) analysis. These studies revealed that CID 129886986 and CID 115269 showed stronger binding affinities with drug-likeness and no toxicity than the FDA-approved standard drug, Donepezil. Additionally, they exhibited strong structural stability with fewer fluctuations throughout the simulation, making them promising candidates for Alzheimer's disease treatment.
Keywords: Alzheimer’s disease; Butyrylcholinesterase; Density functional theory; MM-GBSA; Molecular docking; Molecular dynamics simulations; Pharmacokinetics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
-
- Roy, N., Hassan, A.-M., Alom, R., Rajib, Md. H. R. & Al-Mamun, K. A. The situation of Alzheimer’s disease in Bangladesh: Facilities, expertise, and awareness among general people. J. Neurol. Disord.8, 1–7 (2020).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

