Elevated NEGR1 in brain induces anxiety or depression-like phenotypes and synaptic dysfunction
- PMID: 40382479
- PMCID: PMC12436153
- DOI: 10.1038/s41380-025-03052-7
Elevated NEGR1 in brain induces anxiety or depression-like phenotypes and synaptic dysfunction
Abstract
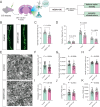
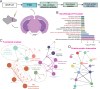
Single nucleotide polymorphisms (SNPs) within 1p31.1 region have shown significant associations with depression, and our prior functional genomics pinpointed a regulatory variant rs3101339 among them. However, its precise role in depression pathogenesis remains elusive. In this study, we employed a series of analytical and functional approaches, including regulatory element annotation, brain expression quantitative trait loci (eQTL), reporter gene assay, electrophoretic mobility shift assay (EMSA), and precise genome editing. Our results confirmed that rs3101339 is a causal variant within 1p31.1 with its risk allele C upregulating NEGR1 expression. To further investigate the consequences of NEGR1 upregulation, we overexpressed NEGR1 in specific region of the mouse brain (including medial prefrontal cortex (mPFC) and ventral hippocampus (vHIP)) using stereotaxic injection. Behavioral assessments revealed that elevated NEGR1 levels in the brain, particularly in the vHIP, resulted in working memory impairment as well as anxiety- and depression-like behaviors in mice. Neuronal sparse labeling assay and transmission electron microscopy revealed that NEGR1 overexpressing in the vHIP leads to dendritic spine loss and synaptic ultrastructure abnormality. Immunoprecipitation-mass spectrometry (IP-MS) further identified 67 high-confidence proteins that interacted with NEGR1, many of which are involved in neurotransmitter exocytosis and synaptic vesicle endocytosis. Transcriptomic profiling revealed 94 differentially expressed genes in NEGR1-OE (vHIP) mice compared to control mice (P adj < 0.05), which were enriched in myelination-related signaling pathways (such as myelination, ensheathment of neurons, axon ensheathment in central nervous system, etc.). Together, our findings implicated that the overexpression of the NEGR1 gene in the mouse brain as a potential driver of anxiety- or depression-like phenotypes potentially through impairing synaptic function and myelination.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Wulsin LR, Vaillant GE, Wells VE. A systematic review of the mortality of depression. Psychosom Med. 1999;61:6–17. - PubMed
-
- James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–858. - PMC - PubMed
-
- Uher R, Payne JL, Pavlova B, Perlis RH. Major depressive disorder in DSM-5: implications for clinical practice and research of changes from DSM-IV. Depress Anxiety. 2014;31:459–71. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

