The efficacy of elevating anandamide via inhibition of fatty acid amide hydrolase (FAAH) combined with internet-delivered cognitive behavioral therapy in the treatment of post-traumatic stress disorder: a randomized, placebo-controlled clinical trial
- PMID: 40382500
- PMCID: PMC12339700
- DOI: 10.1038/s41386-025-02128-w
The efficacy of elevating anandamide via inhibition of fatty acid amide hydrolase (FAAH) combined with internet-delivered cognitive behavioral therapy in the treatment of post-traumatic stress disorder: a randomized, placebo-controlled clinical trial
Abstract
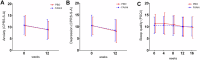
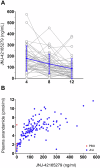
Post-traumatic stress disorder (PTSD) is a severe mental health disorder with limited treatment options. Gold standard treatment includes cognitive behavioral therapies (CBT) that incorporate exposure to traumatic memories to facilitate extinction. CBT can be effective in PTSD, but effects are incomplete and symptoms are prone to spontaneous return. Pharmacologically facilitating fear extinction could potentiate the effects of exposure-based therapy. Here, we explored whether targeting the endocannabinoid (eCB) system, a neuromodulatory system critically involved in fear extinction, would promote the efficacy of exposure-based CBT. Specifically, we tested the effects of elevating the eCB ligand anandamide (AEA) via inhibition of its main degradative enzyme, fatty acid amide hydrolase (FAAH). In this double-blind, placebo-controlled study, patients with PTSD (N = 100; 85 women) were randomized to the FAAH inhibitor (FAAHi) JNJ-42165279 (25 mg b.i.d.) or placebo for 12 weeks. In weeks 5-12, all participants completed an internet-delivered CBT that included exposure-based modules. The primary outcome was clinician-assessed PTSD symptom severity (CAPS-5). Secondary outcomes included self-reported symptoms of PTSD, depression, anxiety, and sleep quality. Blood samples were taken to measure levels of drug and eCBs. Overall, PTSD symptoms improved over time. While FAAHi increased AEA levels, there was no effect of FAAHi on PTSD symptoms or any secondary measure. FAAHi combined with internet-delivered CBT did not improve PTSD symptoms to a greater extent than internet-delivered CBT alone. Thus, FAAH inhibition does not appear to be a suitable adjunct treatment for enhancing CBT in PTSD. This study was registered as Eudra-CT 2020-001965-36.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: MES and DJP were full-time employees of Janssen Pharmaceutica NV during the execution of the study. MH received clinical trial materials and funding from Janssen Pharmaceutica NV for work related to this trial. MH has also received consulting fees, research support or other compensation from Indivior, Camurus, Molteni, BrainsWay, Aelis Farma, and Lundbeck. AJC has received consulting fees from Indivior, Camurus, Lundbeck, and DNEPharma. LMM has received consulting fees from Synendos Therapeutics. All other authors declare no biomedical financial interest or potential conflicts of interest. Dr. Leah Mayo is the NPP Social Media Editor and Drs. Matthew Hill and Markus Heilig are NPP Senior Editors.
Figures
References
-
- Foa EB, Keane TM, Friedman MJ. Effective treatments for PTSD: practice guidelines from the international society for traumatic stress studies. New York, NY: The Guilford Press; 2000:xii, pp. 388.
-
- Hembree EA, Rauch SAM, Foa EB. Beyond the manual: the insider’s guide to prolonged exposure therapy for PTSD. Cogn Behav Pract. 2003;10:22–30. 10.1016/S1077-7229(03)80005-6.
-
- Ruzek JI, Eftekhari A, Rosen CS, Crowley JJ, Kuhn E, Foa EB. Factors related to clinician attitudes toward prolonged exposure therapy for PTSD. J Trauma Stress. 2014;27:423–9. 10.1002/jts.21945. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

