Characteristics and treatment outcome in a prospective cohort of 639 advanced high-grade digestive neuroendocrine neoplasms (NET G3 and NEC). The NORDIC NEC 2 study
- PMID: 40382522
- PMCID: PMC12322073
- DOI: 10.1038/s41416-025-03054-w
Characteristics and treatment outcome in a prospective cohort of 639 advanced high-grade digestive neuroendocrine neoplasms (NET G3 and NEC). The NORDIC NEC 2 study
Erratum in
-
Correction: Characteristics and treatment outcome in a prospective cohort of 639 advanced high-grade digestive neuroendocrine neoplasms (NET G3 and NEC). The NORDIC NEC 2 study.Br J Cancer. 2025 Aug;133(3):420. doi: 10.1038/s41416-025-03095-1. Br J Cancer. 2025. PMID: 40659859 Free PMC article. No abstract available.
Abstract
Background: Digestive high-grade neuroendocrine neoplasms (HG-NEN) are rare and classified as neuroendocrine carcinomas (NEC) or neuroendocrine tumours G3 (NET G3), and differ in clinical and molecular characteristics, response to treatment and prognosis.
Methods: Prospective multicenter study registering clinical data on patients with digestive HG-NEN. Treatment outcome in patients with advanced disease was compared after centralized pathological re-evaluation.
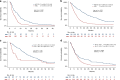
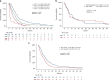
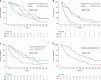
Results: 427 NEC and 117 NET G3 received palliative chemotherapy. Immediate progression rate was 41% and 24%, progression-free survival (PFS) 3.4 m and 7.4 m, overall survival (OS) 7.4 m and 21.8 m for NEC and NET G3, respectively. Significant factors for OS in NEC were performance status (PS), Ki-67 > 55%, alkaline phosphatase (ALP), age, sex and for PFS colorectal primary and PS. NEC Ki-67 < 55% had similar OS comparing treatment. Significant factors for OS in NET G3 were platinum-based treatment, PS, age and ALP, and for PFS platinum-based treatment.
Conclusions: Survival was shorter than expected in this unique population-based cohort of advanced digestive HG-NEN, likely due to inclusion of elderly and patients with poor PS. Several novel prognostic factors were identified for NEC and NET G3. An initial sub-effective platinum-based treatment for NET G3 could not be compensated by later-line treatment.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: ML achieved an unrestricted grant from Scandion Oncology, Denmark. All other authors have declared no conflicts of interest. Ethics approval and consent to participate: The study was conducted according to the Declaration of Helsinki and approved by ethics committees in Norway (REK-vest-2012/940), Sweden (REC-Uppsala-Dnr-2012/285) and Denmark (Region-Hovedstaden-H-4-2012-108). All patients signed written informed consent.
Figures
References
-
- Sorbye H, Grande E, Pavel M, Tesselaar M, Fazio N, Reed NS, et al. European Neuroendocrine Tumor Society (ENETS) 2023 guidance paper for digestive neuroendocrine carcinoma. J Neuroendocrinol. 2023;35:e13249. - PubMed
-
- Heetfeld M, Chougnet CN, Olsen IH, Rinke A, Borbath I, Crespo G, et al. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr-Relat cancer. 2015;22:657–64. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

