FBXL18 promotes endometrial carcinoma progression via destabilizing DUSP16 and thus activating JNK signaling pathway
- PMID: 40382593
- PMCID: PMC12085810
- DOI: 10.1186/s12935-025-03808-9
FBXL18 promotes endometrial carcinoma progression via destabilizing DUSP16 and thus activating JNK signaling pathway
Abstract
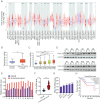
Objective: The therapeutic options for patients with advanced endometrial carcinoma (EC) were still limited and the prognosis remained unfavorable. F-box and leucine-rich repeat protein 18 (FBXL18), belonging to the F-box protein family, was frequently altered in human cancer, while its functional role and underlying mechanisms in EC were largely unexplored.
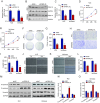
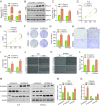
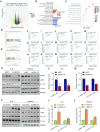
Methods: The expression of FBXL18 in EC tissues and cells were explored using data mining strategies and further experiments. Multiple in vitro assays, including CCK-8, colony formation, wound healing, and Transwell invasion assays, were performed to assess the function of FBXL18 on cell proliferation, migration, and invasion. Bioinformatic analyses, western blot, qRT-PCR, Co-immunoprecipitation and ubiquitination assays were employed to identify the downstream pathway and direct substrate of FBXL18.
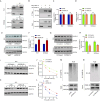
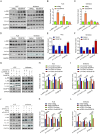
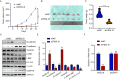
Results: FBXL18 was highly expressed in EC tissues and cell lines, and EC patients with high FBXL18 expression had poor clinical outcome. Loss- and gain-of-function assays showed that silencing FBXL18 suppressed EC cell proliferation, migration, and invasion, while overexpressing FBXL18 caused the opposite effects. Mechanistically, FBXL18 could physically interacted with DUSP16, a dual specificity phosphatase, leading to its ubiquitination and degradation, and thus activating JNK signaling pathway. Upregulation of DUSP16 in EC cells alleviated FBXL18 overexpression-induced activation of JNK signaling pathway, and reversed FBXL18 overexpression-mediated enhanced cell capacities of proliferation, migration, and invasion.
Conclusion: In summary, our study had showcased the elevated expression, prognostic prediction performance, and the malignant tumor-promoting role of FBXL18 in EC. The novel mechanisms underlying this phenotype are that FBXL18 promotes the ubiquitination and degradation of DUSP16, and thus activates JNK/c-JUN signaling to facilitate EC progression.
Keywords: DUSP16; Endometrial carcinoma; FBXL18; JNK signaling; Ubiquitination.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The experiment with patient tissue specimens was authorized by the Ethics Committee of Renmin Hospital of Wuhan University. All animal experiments were approved by the Animal Care and Use Committee of Wuhan University Renmin Hospital. Consent for publication: All authors have approved the publication of this study. Competing interests: The authors declare no competing interests.
Figures
References
-
- Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol. 2014;15(7):e268–278. - PubMed
-
- Huvila J, Pors J, Thompson EF, Gilks CB. Endometrial carcinoma: molecular subtypes, precursors and the role of pathology in early diagnosis. J Pathol. 2021;253(4):355–65. - PubMed
-
- Léon-Castillo A. Update in the molecular classification of endometrial carcinoma. Int J Gynecol Cancer. 2023;33(3):333–42. - PubMed
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Li J, Yang H, Zhang L, Zhang S, Dai Y. Metabolic reprogramming and interventions in endometrial carcinoma. Biomed Pharmacother. 2023;161:114526. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

