The effect of a systematic multi-dimensional assessment in severe uncontrolled asthma: a literature review and protocol for an investigator-initiated, open-label, randomized-controlled trial (EXACT@home study)
- PMID: 40382637
- PMCID: PMC12085824
- DOI: 10.1186/s12890-025-03646-5
The effect of a systematic multi-dimensional assessment in severe uncontrolled asthma: a literature review and protocol for an investigator-initiated, open-label, randomized-controlled trial (EXACT@home study)
Abstract
Introduction: Severe asthma affects 3.6% of the asthma population, in which patients are uncontrolled despite optimal drug therapy and management of treatable traits. These patients are eligible for treatment with biologicals, which provide significant benefits but are costly and need precise indication. However, identifying all individual treatable traits before diagnosing severe asthma is challenging. A systematic multi-dimensional assessment may help identify and address these hidden traits, resulting in tailored treatment and reducing the number of unnecessary biological prescriptions.
Methods: A literature review was conducted to address the knowledge gap on the effectiveness and added value of a systematic assessment and treatment in difficult-to-treat or severe asthma, followed by an outline of a study protocol to implement this in patients diagnosed with severe asthma.
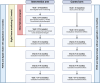
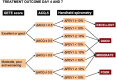
Results: The literature review revealed limited evidence on the effectiveness of systematic assessments in difficult-to-treat or severe asthma, largely due to the use of different study methods and outcome measures. Notably, only one of the selected articles employed a randomized controlled design. To address this gap, the EXpert Asthma Copd Trajectory with digital support (EXACT@home) study was proposed, which aims to improve the assessment and treatment of treatable traits in severe asthma before (re)considering treatment with biologicals. This study uses a prospective, open label, randomized controlled trial design with the primary aim of reducing biological prescriptions. Patients are eligible for inclusion if they have previously been diagnosed with severe uncontrolled asthma with an indication for treatment with biologicals. The intervention arm undergoes a 6-week systematic assessment program targeting treatable traits followed by tailored treatment, while the control arm directly receives treatment with biologicals. Both arms are followed for 12 months with secondary outcomes including asthma control, quality of life and exacerbation frequency.
Discussion: Difficult-to-treat or severe asthma requires tailored treatments based on individual treatable traits, but challenges remain in accurately identifying these traits. Existing literature highlights the beneficial effects of systematic assessments, but conclusive evidence is lacking. The EXACT@home study aims to provide high quality evidence on the effectiveness of such an assessment in the management of severe uncontrolled asthma, addressing a gap in the current literature.
Trial registration: NCT05831566 (Clinicaltrials.gov), registered at 14-04-2023.
Protocol version: version 6, date 27-03-2024.
Keywords: Difficult-to-treat asthma; EHealth; Severe asthma; Systematic assessment; Treatable traits.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethics approval for the EXACT@home study was provided by the Medical research Ethics Committees United (NL79996.100.22). Approval by the Institutional Research Board and Board of Directors of the Franciscus Gasthuis & Vlietland (FGV) hospital was also provided. The study is registered at clinicaltrials.gov (NCT05831566). Informed consent will be obtained from all subjects (supplement 5). Consent for publication: Not applicable. Competing interests: LB: Personal fees and/or speaker fees from Teva, Sanofi. GB: Grants and/or personal fees from AstraZeneca, Teva, Sanofi, GSK, ALK, Novartis, Glaxo Smith Kline, Sanofi, Chiesi. JA: none DB: none JC: Personal fees and/or speaker fees from Astra Zeneca, Sanofi Genzyme, Novartis, Chiesi, GSK MD: none YD: none UF: none EG: none PH: none RH: none MJ: none JK: Grants and/or personal fees from ALK, Chiesi, GSK, Novartis, AstraZeneca, Sanofi, Boerhinger, Teva, Viatris, Stallergen, Abbot, all outside the submitted work. RM: none BO: none MR: none SS: Personal fees and/or speaker fees from Genzyme, Chiesi, GSK, Boehringer Ingelhem, Astra Zeneca YT: none EV: Speaker fees from Boehringer Ingelheim, Sanofi, Astra Zeneca, Periscaldes, Springer Media BV, GSK RW: none ECV: Unconditional research grants from AstraZeneca and Pfizer JV: Unrestricted faculty research grants from GSK, Teva, AZ, Chiesi, Sanofi, and speaker fees from AZ, GSK, Sanofi, Chiesi, Stichting RoLeX and Health Investment.
Figures
References
-
- Global Initiative for Asthma (GINA). Global Strategy for Asthma management and Prevention 2023. Available from: https://ginasthma.org/2023-gina-main-report/.
-
- Hekking PW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. - PubMed
-
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–73. - PubMed
-
- Agusti A, Bel E, Thomas M, Vogelmeier C, Brusselle G, Holgate S, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47(2):410–9. - PubMed
-
- Agusti A, Bafadhel M, Beasley R, Bel EH, Faner R, Gibson PG, et al. Precision medicine in airway diseases: moving to clinical practice. Eur Respir J. 2017;50(4):1701655. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

