Real-world data on the use of the Shingrix vaccine among patients with inflammatory arthritis and risk of cardiovascular events following herpes zoster
- PMID: 40382657
- PMCID: PMC12085024
- DOI: 10.1186/s13075-025-03565-0
Real-world data on the use of the Shingrix vaccine among patients with inflammatory arthritis and risk of cardiovascular events following herpes zoster
Abstract
Background: Risk of cardiovascular events may increase after herpes zoster; therefore, American College of Rheumatology guidelines strongly recommend vaccination against herpes zoster in patients aged ≥ 18 years with rheumatic and musculoskeletal diseases taking immunosuppressive medications. Here, we investigated the effectiveness of Shingrix among patients with inflammatory arthritis and estimated the post-herpes zoster risk of cardiovascular events.
Methods: In this retrospective observational cohort study, data were obtained from the Optum™ Clinformatics™ Data Mart on patients aged ≥ 18 years with rheumatoid arthritis, psoriatic arthritis, or axial spondyloarthritis. The proportions of patients receiving any Shingrix dose, a second dose, and a second dose within 6, 9, and 12 months were calculated. Incidence of herpes zoster following inflammatory arthritis diagnosis was reported. Vaccine effectiveness was calculated as (1 - incidence rate ratio of herpes zoster) × 100. Relative risk of cardiovascular events was assessed independently in the 30-, 45-, 60-, and 90-day periods post-herpes zoster in a subgroup of patients who experienced cardiovascular events.
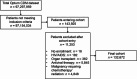
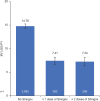
Results: The final cohort included 132,672 patients with inflammatory arthritis. Mean age was 60.4 years, 71.9% were female, and 80.0% were diagnosed with rheumatoid arthritis. Overall, 28,690 (21.6%) patients received ≥ 1 Shingrix dose, of whom only 73.2% received a second dose. Of those receiving a second dose, 17,598 (83.8%) received it within the recommended 2-6 months after the first. Herpes zoster occurred in 4,342 (3.3%) patients, of which 360 cases occurred after Shingrix vaccination. The incidence rate (95% confidence interval) of herpes zoster per 1,000 person-years was 7.41 (6.64, 8.17) after any Shingrix vaccination vs. 14.76 (14.30, 15.22) without vaccination (crude vaccine effectiveness: 50%). The risk of venous thromboembolic events was elevated in the 60-90 days post-herpes zoster; no significantly increased risk was observed for any other cardiovascular events.
Conclusions: This study showed that the effectiveness of Shingrix in patients with inflammatory arthritis on immunomodulatory treatment was 50%, and the risk of venous thromboembolic events was increased in the 60-90 days after herpes zoster, supporting the recommendation that adults with inflammatory arthritis should receive vaccination against herpes zoster to reduce the risk of such events.
Keywords: Arthritis; Cardiovascular disease; Herpes zoster; Inflammatory disease; Real-world studies; Vaccines.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: J.C. has received research grants and consulting fees from AbbVie, Amgen, Bendcare, Bristol Myers Squibb, Janssen, Lilly, Novartis, Pfizer, Sanofi/Regeneron, and UCB. D.C., W.K., and A.G. are full-time employees of AbbVie and may hold AbbVie stock or stock options. K.W. has received consulting fees and/or research grants from AbbVie, Bristol Myers Squibb, Galapagos, Gilead, Lilly, Pfizer, Roche, and UCB.
Figures

Similar articles
-
Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases.JAMA. 2012 Jul 4;308(1):43-9. doi: 10.1001/jama.2012.7304. JAMA. 2012. PMID: 22760290 Free PMC article.
-
The use, safety, and effectiveness of herpes zoster vaccination in individuals with inflammatory and autoimmune diseases: a longitudinal observational study.Arthritis Res Ther. 2011;13(5):R174. doi: 10.1186/ar3497. Epub 2011 Oct 24. Arthritis Res Ther. 2011. PMID: 22024532 Free PMC article.
-
Recombinant Zoster Vaccine (Shingrix): Real-World Effectiveness in the First 2 Years Post-Licensure.Clin Infect Dis. 2021 Sep 15;73(6):941-948. doi: 10.1093/cid/ciab125. Clin Infect Dis. 2021. PMID: 33580242
-
An Analysis of Spontaneously Reported Data of Vesicular and Bullous Cutaneous Eruptions Occurring Following Vaccination with the Adjuvanted Recombinant Zoster Vaccine.Drug Saf. 2021 Dec;44(12):1341-1353. doi: 10.1007/s40264-021-01118-3. Epub 2021 Oct 7. Drug Saf. 2021. PMID: 34622421 Free PMC article. Review.
-
Vaccines for preventing herpes zoster in older adults.Cochrane Database Syst Rev. 2019 Nov 7;2019(11):CD008858. doi: 10.1002/14651858.CD008858.pub4. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2023 Oct 2;10:CD008858. doi: 10.1002/14651858.CD008858.pub5. PMID: 31696946 Free PMC article. Updated.
Cited by
-
Analyzing Molecular Determinants of Nanodrugs' Cytotoxic Effects.Int J Mol Sci. 2025 Jul 11;26(14):6687. doi: 10.3390/ijms26146687. Int J Mol Sci. 2025. PMID: 40724938 Free PMC article.
References
-
- Weinberg JM. Herpes zoster: epidemiology, natural history, and common complications. J Am Acad Dermatol. 2007;57(Suppl 6):S130–5. - PubMed
-
- Sullivan KM, Farraye FA, Winthrop KL, Willer DO, Vink P, Tavares-Da-Silva F. Safety and efficacy of recombinant and live herpes zoster vaccines for prevention in at-risk adults with chronic diseases and immunocompromising conditions. Vaccine. 2023;41(1):36–48. - PubMed
-
- Centers for Disease Control and Prevention (CDC). Update on herpes zoster vaccine: licensure for persons aged 50 through 59 years. MMWR Morb Mortal Wkly Rep. 2011;60(44):1528. - PubMed
-
- Holcomb K, Weinberg JM. A novel vaccine (Zostavax) to prevent herpes zoster and postherpetic neuralgia. J Drugs Dermatol. 2006;5(9):863–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

