KRAS inhibition reverses chemotherapy resistance promoted by therapy-induced senescence-like in pancreatic ductal adenocarcinoma
- PMID: 40382842
- PMCID: PMC12143771
- DOI: 10.1016/j.tranon.2025.102421
KRAS inhibition reverses chemotherapy resistance promoted by therapy-induced senescence-like in pancreatic ductal adenocarcinoma
Abstract
Background: Emerging evidence suggests that chemotherapy can accumulate senescent-like cells within tumor tissues, a phenomenon linked to therapy resistance. The aim of this study is to analyze the senescence-like state of after-treatment persistent cells associated with KRAS mutational status to offer a therapeutic strategy to target these cells in pancreatic ductal adenocarcinoma (PDAC).
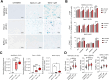
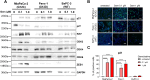
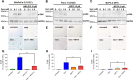
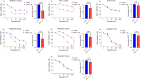
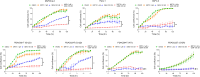
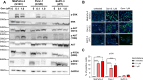
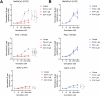
Experimental design: Three commercial cell lines and five patient-derived primary cell cultures with different KRAS statuses were studied following gemcitabine treatment. Senescence-like status was assessed using SA-β-gal, together with cell cycle regulators such as p21. Additionally, KRAS mutations were modulated using MRTX1133 and AMG-510, and the signaling pathways ERK and AKT were analyzed and modulated in vitro. Finally, p21 expression, associated with the senescence-like state, on patient outcomes and treatment response was analyzed in publicly available bulk RNA-seq and single-nucleus datasets.
Results: We observed an overexpression of p21 alongside an increase in SA-β-gal signal in response to gemcitabine treatment, indicating the induction of a senescence-like state. Specific inhibition of KRAS G12D or G12C mutations reduced SA-β-gal signal and sensitized PDAC cells to gemcitabine. Moreover, ERK inhibition but not AKT inhibition decreased SA-β-gal signal. Additionally, we characterized p21 expression levels in relation to patient outcomes and found that they are modulated by treatment.
Conclusions: This dual-targeted therapeutic strategy holds promises for overcoming the challenges posed by KRAS-driven cancers, particularly in addressing the formidable obstacle of pancreatic cancer.
Keywords: Gemcitabine; MRTX1133; Mutated KRAS; PDAC; Resistance; Senescence-like.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest No potential conflicts of interest were disclosed. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Neoptolemos J.P., Kleeff J., Michl P., Costello E., Greenhalf W., Palmer D.H. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018;15:333–348. - PubMed
-
- Conroy T., Hammel P., Hebbar M., Ben Abdelghani M., Wei A.C., Raoul J.L., et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N. Engl. J. Med. 2018;379:2395–2406. - PubMed
-
- Fassnacht M., Terzolo M., Allolio B., Baudin E., Haak H., Berruti A., et al. Combination chemotherapy in advanced adrenocortical carcinoma. N. Engl. J. Med. 2012;366:2189–2197. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

