Light-powered phagocytic macrophage microrobot (phagobot): both in vitro and in vivo
- PMID: 40383739
- PMCID: PMC12086205
- DOI: 10.1038/s41377-025-01881-3
Light-powered phagocytic macrophage microrobot (phagobot): both in vitro and in vivo
Abstract
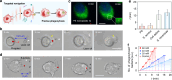
Micro/nanorobots based on immune cells show great potential for addressing challenging biological and biomedical conditions. However, their powerful innate immune functions, particularly the phagocytosis capabilities, remain a big challenge to fully leverage with the current designs of immune cell-based microrobots. Herein, we report a light-powered phagocytic macrophage microrobot (phagobot), which is capable of robotic navigation toward specific foreign bio-threats and executing precise phagocytosis of these targeted entities under light control. Without genetic modification or nanoengineering of macrophages, the phagobot's "wake-up" program is achieved through direct activation of a resting-state macrophage by a tightly focused near-infrared (NIR) light beam. The phagobot exhibits robotic steering and directional navigation controlled by optical manipulation of the extended pseudopodia within the activated macrophage. It can further execute targeted phagocytic clearance tasks via engulfing various foreign bio-threats, including nanoplastics, microbials, and cancer cell debris. Notably, the phagobot can be constructed in a living larval zebrafish through optical activation and manipulation of the endogenous macrophage, which also exhibits controllable navigation and targeted phagocytic capabilities in vivo. With the intrinsic immune functions of macrophages, our light-powered phagobot represents a novel form of intelligent immune cell-based microrobots, holding many new possibilities for precise immune regulation and treatment for immune-related diseases.
© 2025. The Author(s).
Conflict of interest statement
Conflict of interest: The authors declare no competing interests.
Figures




Similar articles
-
Magnetically Powered Immunogenic Macrophage Microrobots for Targeted Multimodal Cancer Therapy.Small. 2023 Oct;19(42):e2301489. doi: 10.1002/smll.202301489. Epub 2023 Jun 10. Small. 2023. PMID: 37300342
-
Light-controlled soft bio-microrobot.Light Sci Appl. 2024 Feb 26;13(1):55. doi: 10.1038/s41377-024-01405-5. Light Sci Appl. 2024. PMID: 38403642 Free PMC article.
-
Magnetically Manipulated Optoelectronic Hybrid Microrobots for Optically Targeted Non-Genetic Neuromodulation.Adv Mater. 2024 Feb;36(8):e2305632. doi: 10.1002/adma.202305632. Epub 2023 Dec 8. Adv Mater. 2024. PMID: 37805826
-
Macrophage-Based Microrobots for Anticancer Therapy: Recent Progress and Future Perspectives.Biomimetics (Basel). 2023 Nov 18;8(7):553. doi: 10.3390/biomimetics8070553. Biomimetics (Basel). 2023. PMID: 37999194 Free PMC article. Review.
-
Light-Driven Microrobots for Targeted Drug Delivery.ACS Biomater Sci Eng. 2024 Sep 9;10(9):5562-5594. doi: 10.1021/acsbiomaterials.4c01191. Epub 2024 Aug 15. ACS Biomater Sci Eng. 2024. PMID: 39147594 Review.
References
-
- Soto, F. et al. Smart materials for microrobots. Chem. Rev.122, 5365–5403 (2022). - PubMed
-
- Xiong, J. Y. et al. Photonic nanojet-regulated soft microalga-robot with controllable deformation and navigation capability. PhotoniX5, 43 (2024).
-
- Pan, T. et al. Bio‐micromotor tweezers for noninvasive bio‐cargo delivery and precise therapy. Adv. Funct. Mater.32, 2111038 (2022).
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

