From blood drops to biomarkers: a scoping review of microsampling in mass spectrometry-based proteomics
- PMID: 40383761
- PMCID: PMC12085825
- DOI: 10.1186/s12014-025-09540-w
From blood drops to biomarkers: a scoping review of microsampling in mass spectrometry-based proteomics
Abstract
Background: Microsamples are simple blood sampling procedures utilizing small blood draws. Although microsamples are regularly used in some disciplines, proteomic analysis of these samples is an emerging field. Currently, it is unclear whether the quantitative precision and proteome coverage achieved in microsamples is comparable to plasma or serum. As a consequence, microsamples are not used in proteomics to the same degree as more traditional blood samples.
Objectives: The objective of this scoping review was to report the applications of microsamples within clinical mass spectrometry-based proteomics. This was accomplished by describing both proof-of-concept and clinical proteomics research within this field, with an additional evaluation of the newest advances regarding clinical proteomics.
Inclusion criteria: Original scientific literature was included where bottom-up mass spectrometry was used to analyze endogenous proteins from human microsamples.
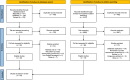
Methods: Relevant publications were sourced through three scientific databases (MEDLINE, EMBASE and Scopus) in addition to backward and forward citation searches through Scopus. Record screening was performed independently by two separate authors. The review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) guidelines.
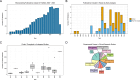
Results: A total of 209 records were screened for inclusion from database searches and 3157 records were screened from forward and backward citation searches, resulting in 64 eligible studies. An evaluation of proof-of-concept research within this field revealed that although microsamples are amenable to high-throughput proteomics using a variety of targeted and untargeted acquisition methods, quantification remained a relevant issue. Microsampling practices were heterogeneous, and no standard procedure existed for protein quantification. Clinical studies investigated protein expression in numerous disease or experimental groups, including hemoglobinopathies and immunodeficiency disorders.
Conclusion: The use of microsamples is increasing within the proteomics field and these samples are amenable to standard bottom-up workflows. Although microsamples present a clear advantage in terms of sampling procedure, both the sample collection and quantification procedures remain to be standardized. However, there is an incentive to address the remaining issues, since microsampling would greatly reduce the resources necessary to sample large cohorts within clinical proteomics, a field that currently lacks large discovery and validation cohorts.
Keywords: Clinical proteomics; Dried blood spots; Mass spectrometry; Microsampling; Scoping review.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- FDA-NIH Biomarker Working Group BEST (Biomarkers, EndpointS, and other Tools) Resource. Silver Spring (MD), Bethesda (MD): Food and Drug Administration (US), National Institutes of Health (US). 2016. https://www.ncbi.nlm.nih.gov/pubmed/27010052. [Accessed February 15, 2025] - PubMed
-
- Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1(11):845–67. - PubMed
-
- Therrell BL, Padilla CD, Loeber JG, Kneisser I, Saadallah A, Borrajo GJC, et al. Current status of newborn screening worldwide: 2015. Semin Perinatol. 2015;39(3):171–87. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
