Vascular endothelial growth factor signaling in health and disease: from molecular mechanisms to therapeutic perspectives
- PMID: 40383803
- PMCID: PMC12086256
- DOI: 10.1038/s41392-025-02249-0
Vascular endothelial growth factor signaling in health and disease: from molecular mechanisms to therapeutic perspectives
Abstract
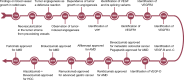
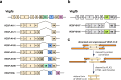
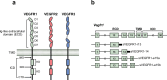
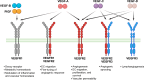
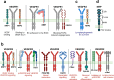
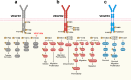
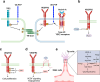
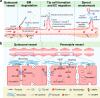
Vascular endothelial growth factor (VEGF) signaling is a critical regulator of vasculogenesis, angiogenesis, and lymphangiogenesis, processes that are vital for the development of vascular and lymphatic systems, tissue repair, and the maintenance of homeostasis. VEGF ligands and their receptors orchestrate endothelial cell proliferation, migration, and survival, playing a pivotal role in dynamic vascular remodeling. Dysregulated VEGF signaling drives diverse pathological conditions, including tumor angiogenesis, cardiovascular diseases, and ocular disorders. Excessive VEGF activity promotes tumor growth, invasion, and metastasis, while insufficient signaling contributes to impaired wound healing and ischemic diseases. VEGF-targeted therapies, such as monoclonal antibodies and tyrosine kinase inhibitors, have revolutionized the treatment of diseases involving pathological angiogenesis, offering significant clinical benefits in oncology and ophthalmology. These therapies inhibit angiogenesis and slow disease progression, but they often face challenges such as therapeutic resistance, suboptimal efficacy, and adverse effects. To further explore these issues, this review provides a comprehensive overview of VEGF ligands and receptors, elucidating their molecular mechanisms and regulatory networks. It evaluates the latest progress in VEGF-targeted therapies and examines strategies to address current challenges, such as resistance mechanisms. Moreover, the discussion includes emerging therapeutic strategies such as innovative drug delivery systems and combination therapies, highlighting the continuous efforts to improve the effectiveness and safety of VEGF-targeted treatments. This review highlights the translational potential of recent discoveries in VEGF biology for improving patient outcomes.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Carmeliet, P. Angiogenesis in life, disease and medicine. Nature438, 932–936 (2005). - PubMed
-
- Ferrara, N. & Kerbel, R. S. Angiogenesis as a therapeutic target. Nature438, 967–974 (2005). - PubMed
-
- Stephenson, J. A., Goddard, J. C., Al-Taan, O., Dennison, A. R. & Morgan, B. Tumour angiogenesis: a growth area—from John Hunter to Judah Folkman and beyond. J. Cancer Res.2013, 895019 (2013).
-
- Lenzi, P., Bocci, G. & Natale, G. John Hunter and the origin of the term “angiogenesis”. Angiogenesis19, 255–256 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NRF-2021R1A6A1A03038890/National Research Foundation of Korea (NRF)
- RS-2023-00268276/National Research Foundation of Korea (NRF)
- 2021R1A6C101A564/Ministry of Education (Ministry of Education of the Republic of Korea)
- RS-2024-00436674/Ministry of Education (Ministry of Education of the Republic of Korea)
- RS-2024-00403190/Ministry of Trade, Industry and Energy (Ministry of Trade, Industry and Energy, Korea)
LinkOut - more resources
Full Text Sources

