ZC3H12D gene expression exhibits dual effects on the development and progression of lung adenocarcinoma
- PMID: 40383849
- PMCID: PMC12086231
- DOI: 10.1038/s41598-025-02163-z
ZC3H12D gene expression exhibits dual effects on the development and progression of lung adenocarcinoma
Abstract
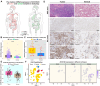
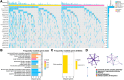
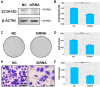
While precision oncology requires robust biomarkers, current predictors for lung adenocarcinoma (LUAD) often show limited clinical utility. This study investigates the multifaceted roles of ZC3H12D, a novel immunomodulatory molecule, in LUAD progression and tumor microenvironment regulation. Multi-omics analyses integrated ZC3H12D transcriptomic (511 tumors vs 59 normals), proteomic (74 tumors vs 69 normals), and single-cell RNA-seq data (15 tumors vs 11 normals). Immunohistochemistry validated ZC3H12D expression in 51 matched pairs. Computational biology approaches assessed immune infiltration, genomic instability (TMB/MSI/HRD), and pathway enrichment. Functional validation employed ZC3H12D knockdown in PC9 cells with colony formation and transwell assays. Multi-omics verification confirmed ZC3H12D upregulation in LUAD at both mRNA and protein levels (p < 0.001), with single-cell resolution revealing predominant localization in tumor-infiltrating immune cells. Moreover, ZC3H12D expression positively correlated with immune regulatory genes while inversely associating with genes involved in cellular respiration. Its expression was also linked to clinical markers such as TMB, MSI, HRD, tumor purity, and ploidy. Notably, high ZC3H12D expression revealed Immune-infiltrated microenvironment and favorable prognosis, despite silencing ZC3H12D resulted in significant inhibition of tumor cell proliferation and invasion in vitro (p < 0.001). Our findings demonstrate that high ZC3H12D expression in immune cells appears to enhance antitumor immune activity, whereas lower expression in malignant cells contributes to reduced cellular proliferation and migration. This spatial duality challenges conventional biomarker paradigms and provides mechanistic insights for developing cell type-targeted therapies.
Keywords: ZC3H12D; Cell migration; Cell proliferation; Dual effects; Lung cancer.
© 2025. The Author(s).
Conflict of interest statement
Declaration. Competing interests: Yuhuan Zhang and Jieyi Li is employed by the Jiaxing Yunying Medical Inspection Co., Ltd. and the Shanghai Yunying Biopharmaceutical Technology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest. Other Authors have no Competing interest. Ethics approval: This retrospective study was conducted according to the principles of the Declaration of Helsinki by the World Medical Association. The study design was approved specifically by the Internal Review Board of the Zhejiang Cancer Hospital (Reference: IRB-2020–302). All participants who provided tissue specimens provided signed and informed consent for this retrospective study.
Figures



References
-
- Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin.72(1), 7–33. 10.3322/caac.21708 (2022). - PubMed
-
- Thai, A. A., Solomon, B. J., Sequist, L. V., Gainor, J. F. & Heist, R. S. Lung cancer. Lancet398(10299), 535–554. 10.1016/S0140-6736(21)00312-3 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

