CPA1 S282P mutation leads to chronic pancreatitis in rabbits
- PMID: 40383969
- PMCID: PMC12361900
- DOI: 10.24272/j.issn.2095-8137.2024.419
CPA1 S282P mutation leads to chronic pancreatitis in rabbits
Abstract
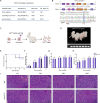
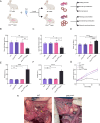
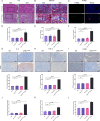
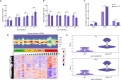
Chronic pancreatitis (CP) is a progressive and irreversible fibroinflammatory disease that markedly increases susceptibility to pancreatic cancer and remains without effective targeted therapies. Among the genetic contributors to CP, the carboxypeptidase A1 p.Ser282Pro ( CPA1 S282P ) variant has been proposed to promote disease through misfolding-induced endoplasmic reticulum stress (ERS), although the broader pathogenic landscape remains incompletely defined. This study generated a rabbit model mimicking the human CPA1 S282P mutation using the SpRY-ABE-8.17 system. Homozygous CPA1 S282P rabbits exhibited characteristic human CP phenotypes following alcohol induction, including visceral pain, elevated serum lipase and amylase, inflammatory cell infiltration, and extensive pancreatic fibrosis. Biochemical analyses confirmed that the p.S282P mutation induced CPA1 misfolding and elevated the expression of ERS markers GRP78 and CHOP in both transfected HEK293T cells and homozygous mutant rabbits. Notably, the CPA1 S282P mutation markedly disrupted intra-pancreatic lipid homeostasis, contributing to the development of CP in mutant rabbits. This study successfully established the first rabbit model of CP that accurately recapitulates CP caused by a defined human point mutation. Additionally, this study provides insights into a previously unrecognized link between CPA1 and intra-pancreatic lipid metabolism, offering a foundation for identifying novel therapeutic targets for human CP.
慢性胰腺炎是一种进行性、不可逆的炎症和纤维化疾病,会增加罹患胰腺癌的风险,目前尚无特定治疗方法。羧肽酶A1 p.Ser282Pro ( CPA1 S282P ) 变体与慢性胰腺炎有关,可能涉及错误折叠引起的内质网应激,尽管其他机制仍未得到充分探索。我们使用SpRY-ABE-8.17系统生成了一个兔模型来模拟人类 CPA1 S282P 突变。 CPA1 S282P 纯合兔在酒精诱导后表现出了特征性的人类慢性胰腺炎表型,包括内脏痛、血清胰腺脂肪酶和淀粉酶升高、炎性浸润和纤维化。重要的是, CPA1 S282P 诱导 CPA1错误折叠并导致HEK 293T细胞和纯合突变兔中内质网应激标记GRP78和CHOP表达升高。进一步研究表明, CPA1 S282P 突变扰乱了胰腺内脂质代谢,导致 CPA1 S282P 兔在酒精诱导后发生慢性胰腺炎。该研究提出了第一个准确复制人类观察到的特定点突变的慢性胰腺炎兔模型。此外,该研究进一步深入了解了 CPA1与胰腺内脂质代谢之间的联系,为开发人类慢性胰腺炎的新治疗靶点提供了宝贵信息。.
Keywords: Carboxypeptidase A1; Chronic pancreatitis; Lipid metabolism; Point mutation; Rabbit.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
