Experimental Autoimmune Neuritis Nerve Demyelination Is Attenuated by Blocking JAK2/STAT3 Signaling Pathway in Rats
- PMID: 40383991
- PMCID: PMC12086307
- DOI: 10.1002/brb3.70566
Experimental Autoimmune Neuritis Nerve Demyelination Is Attenuated by Blocking JAK2/STAT3 Signaling Pathway in Rats
Abstract
Background: Guillain‒Barré syndrome (GBS) is an immune-mediated peripheral neuropathy in which inflammatory cells and cytokines participate. The JAK-STAT signaling pathway is a major pathway involved in cytokine signal transduction, but the role of this pathway in GBS is not clear. AG490 is a tyrosine kinase inhibitor that specifically inhibits JAK2 activity and downregulates STAT3 phosphorylation. The aim of this study was to investigate the function of the JAK2/STAT3 pathway in a rat model of experimental autoimmune neuritis (EAN).
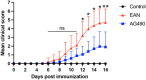
Methods: Lewis rats were divided into three groups: the control, the EAN, and the AG490 groups. The EAN and AG490 groups were immunized with P2 peptide to create the EAN models, while the control group received an equal volume of vehicle solution without P2 peptide. Starting from Day 5 post-immunization (PI), the AG490 group was administered AG490 (10 mg/kg) every other day, while the control and EAN groups received an equal volume of vehicle solution without AG490. All rats were weighed and evaluated according to the EAN function score (1-10) by two investigators. Rats were sacrificed on Day 16 PI, and the sciatic nerves were examined by light microscopy, indirect immunohistochemistry, and western blotting.
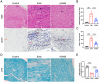
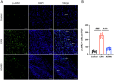
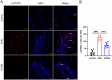
Results: AG490-treated rats had improved clinical scores compared with those of EAN rats. Hematoxylin and eosin (H&E) and CD45 staining showed significant inflammatory infiltration of the sciatic nerve in the EAN group compared with the control group, and demonstrated reduced inflammatory infiltration in the AG490 group. Luxol fast blue (LFB) staining showed a reduction of myelin loss in the AG490 group compared with the EAN group. The levels of TGF-β1, IFN-γ, and IL-6 increased in the EAN group and showed a significant decrease in rats treated with AG490. The JAK2-STAT3 signaling pathway was activated in EAN rats, and the AG490 group showed decreased expression levels of JAK2, p-JAK2, and p-STAT3 compared with those of the EAN group. Immunofluorescence also showed a decrease in the levels of p-JAK2 and p-STAT3 in the sciatic nerve of EAN rats.
Conclusions: The JAK2/STAT3 signaling pathway is involved in the pathogenesis of EAN, and inhibition of this pathway can reduce the inflammatory response in EAN rats. Despite the limitations in extrapolating EAN findings to human GBS, this study provided new insights into the pathogenesis and potential therapeutic targets of human GBS.
Keywords: AG490; JAK‐STAT signaling pathway; experimental autoimmune neuritis; treatment.
© 2025 The Author(s). Brain and Behavior published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Inflammation and proinflammatory cytokine production, but no demyelination of facial nerves, in experimental autoimmune neuritis in Lewis rats.J Neuroimmunol. 2003 Jul;140(1-2):97-101. doi: 10.1016/s0165-5728(03)00178-4. J Neuroimmunol. 2003. PMID: 12864976
-
[Expression of glycolytic genes in immune cells and changes of related immune cells in experimental autoimmune neuritis].Zhonghua Yi Xue Za Zhi. 2023 May 9;103(17):1334-1339. doi: 10.3760/cma.j.cn112137-20220904-01869. Zhonghua Yi Xue Za Zhi. 2023. PMID: 37150684 Chinese.
-
The dynamic expression of canonical Wnt/β-catenin signalling pathway in the pathologic process of experimental autoimmune neuritis.Int J Neurosci. 2020 Nov;130(11):1109-1117. doi: 10.1080/00207454.2020.1725511. Epub 2020 Feb 10. Int J Neurosci. 2020. PMID: 32009498
-
High mobility group box 1 induces the activation of the Janus kinase 2 and signal transducer and activator of transcription 3 (JAK2/STAT3) signaling pathway in pancreatic acinar cells in rats, while AG490 and rapamycin inhibit their activation.Bosn J Basic Med Sci. 2016 Nov 10;16(4):307-312. doi: 10.17305/bjbms.2016.1442. Bosn J Basic Med Sci. 2016. PMID: 27754827 Free PMC article.
-
Experimental allergic neuritis (EAN) as a model for the immune-mediated demyelinating neuropathies.Rev Neurol (Paris). 1996 May;152(5):328-32. Rev Neurol (Paris). 1996. PMID: 8881424 Review.
References
-
- Asbury, A. K. , and McKhann G. M.. 1997. “Changing Views of Guillain‐Barré Syndrome.” Annals of Neurology 41, no. 3: 287–288. - PubMed
-
- Brostoff, S. W. , Levit S., and Powers J. M.. 1977. “Induction of Experimental Allergic Neuritis With a Peptide From Myelin P2 Basic Protein.” Nature 268, no. 5622: 752–753. - PubMed
-
- Chang, K. H. , Lyu R. K., Ro Y. S., et al. 2016. “Increased Serum Concentrations of Transforming Growth Factor‐β1 (TGF‐β1) in Patients With Guillain‐Barré Syndrome.” Clinica Chimica Acta 461: 8–13. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

