Integrins and NAFLD-associated liver diseases: clinical associations, pathophysiological mechanisms and pharmacological implications
- PMID: 40384047
- PMCID: PMC11659783
- DOI: 10.3724/abbs.2024149
Integrins and NAFLD-associated liver diseases: clinical associations, pathophysiological mechanisms and pharmacological implications
Abstract
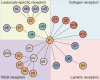
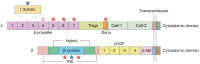
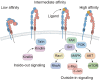
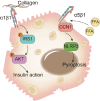
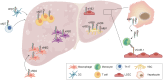
Nonalcoholic fatty liver disease (NAFLD) is a leading cause of chronic liver disease and poses a substantial health burden with increasing incidence globally. NAFLD encompasses a spectrum extending from hepatic steatosis to nonalcoholic steatohepatitis (NASH), with the possibility of progressing to cirrhosis or, in severe instances, hepatocellular carcinoma (HCC). NAFLD extends beyond simple metabolic disruption and involves multiple immune cell-mediated inflammatory processes. Integrins are a family of heterodimeric transmembrane cell adhesion receptors that regulate various aspects of NAFLD onset and progression, including hepatocellular steatosis, hepatic stellate cell (HSC) activation and immune cell infiltration. In this review, we comprehensively summarize the involvement of integrins in NAFLD, as well as the downstream signal transduction mediated by these receptors. Furthermore, we present the latest clinical and preclinical findings on drugs that target integrins for steatosis, inflammation, fibrosis and NAFLD-related HCC treatment.
Keywords: NAFLD; NAFLD-related HCC; NASH; diagnostics and treatments; integrins.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. . 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. - DOI - PubMed
-
- Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, Colombo M, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. . 2018;69:896–904. doi: 10.1016/j.jhep.2018.05.036. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

