Downstream Signaling of Muscarinic M4 Receptors Is Regulated by Receptor Density and Cellular Environment
- PMID: 40384150
- PMCID: PMC12086361
- DOI: 10.1002/prp2.70123
Downstream Signaling of Muscarinic M4 Receptors Is Regulated by Receptor Density and Cellular Environment
Abstract
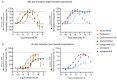
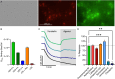
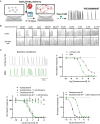
Multiple muscarinic M4 receptor modulators are currently advancing in clinical development for the treatment of positive symptoms in schizophrenia, including agonists and positive allosteric modulators. Considering the importance of comprehending M4 receptor pharmacology for these therapeutic applications, this study investigates M4 receptor signaling pathways upon activation by structurally diverse muscarinic agonists, exploring the role of receptor expression levels and cellular environment on downstream signaling. HEK-293 cells and rat primary neurons expressing human M4 receptors were used to measure the kinetics of cAMP levels and compound effects on neuronal network activity. Receptor expression levels were controlled by a Tet-On system and quantified using a radioactive binding assay. Our findings revealed that most agonists caused a concentration-dependent reduction of cAMP levels (Gi/o) at low concentrations, while inducing an increase in cAMP at higher concentrations (Gs). A less prominent coupling via Gs was observed when receptor density in HEK-293 cells was reduced. In the neuronal assay, most compounds showed consistent inhibition of neuronal activity. A distinct group of agonists displayed a specific profile, with no Gs coupling at high receptor density, partial activation at low receptor density, and low to no effects in the neuronal assay. This study provides a side-by-side comparison of the activity of structurally diverse M4 agonists and highlights compound-specific activation of GPCR intracellular signaling pathways. The data offer new insights into M4 receptor pharmacology that may aid in the development of novel therapies for the treatment of psychiatric diseases.
© 2025 Boehringer Ingelheim Pharma GmbH & Co. KG. Pharmacology Research & Perspectives published by British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics and John Wiley & Sons Ltd.
Conflict of interest statement
M.M., M.P., O.H., U.G., and D.U. are employees of Boehringer Ingelheim Pharma GmbH & Co. KG. All other authors declare no potential conflicts of interest.
Figures




References
-
- Caulfield M. P. and Birdsall N. J., “International Union of Pharmacology. XVII. Classification of Muscarinic Acetylcholine Receptors,” Pharmacological Reviews 50 (1998): 279–290. - PubMed
-
- Brown D. A., “Muscarinic Acetylcholine Receptors (mAChRs) in the Nervous System: Some Functions and Mechanisms,” Journal of Molecular Neuroscience 41 (2010): 340–346. - PubMed
-
- Thiele A., “Muscarinic Signaling in the Brain,” Annual Review of Neuroscience 36 (2013): 271–294. - PubMed
-
- Felder C. C., Goldsmith P. J., Jackson K., et al., “Current Status of Muscarinic M1 and M4 Receptors as Drug Targets for Neurodegenerative Diseases,” Neuropharmacology 136 (2018): 449–458. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

