Defining Biological Variability, Analytical Precision and Quantitative Biophysiochemical Characterization of Human Urinary Extracellular Vesicles
- PMID: 40384173
- PMCID: PMC12086329
- DOI: 10.1002/jev2.70087
Defining Biological Variability, Analytical Precision and Quantitative Biophysiochemical Characterization of Human Urinary Extracellular Vesicles
Abstract
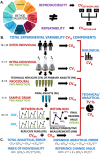
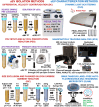
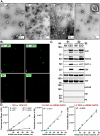
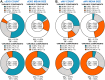
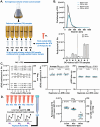
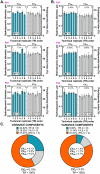
The magnitude of combined analytical errors of urinary extracellular vesicle (uEV) preparation and measurement techniques (CVA) has not been thoroughly investigated to determine whether it exceeds biological variations. We utilized technical replicates of human urine to assess the repeatability of uEV concentration and size measurements by nanoparticle tracking analysis (NTA) following differential velocity centrifugation (DC), silicon carbide, or polyethylene glycol uEV isolation methods. The DC method attained the highest precision. Consequently, DC-derived uEV size, most abundant protein levels, and optical redox ratio (ORR) were further assessed by dynamic light scattering (DLS), immunoblotting or multi-photon (SLAM) microscopy. Procedural errors primarily affected uEV counting and uEV-associated protein quantification, while instrumental errors contributed most to the total variability of NTA- and DLS-mediated uEV sizing processes. The intra-individual variability (CVI) of uEV counts assessed by NTA was smaller than inter-individual variability (CVG), resulting in an estimated index of individuality IOI < 0.6, suggesting that personalized reference interval (RI) is more suitable for interpretation of changes in subject's test results. Population-based RI was more appropriate for ORR (IOI > 1.4). The analytical performance of DC-NTA and DC-SLAM techniques met optimal CVA < 0.5 × CVI criteria, indicating their suitability for further testing in clinical laboratory settings.
Keywords: analytical precision; biological variability; biomarker quantitation; biophysiochemical properties; coefficient of variation; repeatability; urinary extracellular vesicles.
© 2025 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Single Extracellular Vesicle Analysis Performed by Imaging Flow Cytometry and Nanoparticle Tracking Analysis Evaluate the Accuracy of Urinary Extracellular Vesicle Preparation Techniques Differently.Int J Mol Sci. 2021 Nov 18;22(22):12436. doi: 10.3390/ijms222212436. Int J Mol Sci. 2021. PMID: 34830318 Free PMC article.
-
Comparing Approaches to Normalize, Quantify, and Characterize Urinary Extracellular Vesicles.J Am Soc Nephrol. 2021 May 3;32(5):1210-1226. doi: 10.1681/ASN.2020081142. Epub 2021 Mar 29. J Am Soc Nephrol. 2021. PMID: 33782168 Free PMC article.
-
Urinary extracellular vesicles: Assessment of pre-analytical variables and development of a quality control with focus on transcriptomic biomarker research.J Extracell Vesicles. 2021 Oct;10(12):e12158. doi: 10.1002/jev2.12158. J Extracell Vesicles. 2021. PMID: 34651466 Free PMC article.
-
Preparation and characterization of extracellular vesicles.Am J Reprod Immunol. 2021 Feb;85(2):e13367. doi: 10.1111/aji.13367. Epub 2020 Nov 16. Am J Reprod Immunol. 2021. PMID: 33118232 Review.
-
A Method to Analyze Urinary Extracellular Vesicles.Anal Sci. 2020 Jul 10;36(7):791-798. doi: 10.2116/analsci.20R001. Epub 2020 Mar 13. Anal Sci. 2020. PMID: 32173678 Review.
References
-
- Analytical Methods Committee Amctb N . 2015. “An Analyst's Guide to Precision.” Analytical Methods 7, no. 20: 8508–8510. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

