Formation of a β-Endorphin Corona Mitigates Alzheimer's Amyloidogenesis
- PMID: 40384186
- PMCID: PMC12232254
- DOI: 10.1002/smll.202409392
Formation of a β-Endorphin Corona Mitigates Alzheimer's Amyloidogenesis
Abstract
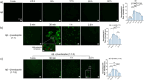
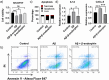
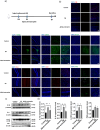
Senile plaques, comprised of nanosized aggregates of amyloid-β (Aβ) peptides in the brain, are a pathological hallmark of Alzheimer's disease (AD). On the other hand, regular physical exercise is known to significantly reduce the risk of developing AD. Here, it is reported on the transformation and toxicity mitigation of Aβ amyloid aggregation by a spontaneous "corona" of β-endorphin, a major peptide hormone released upon exercise to suppress post-exercise pain. Given that both Aβ and β-endorphin co-localize extracellularly in the brain, it is postulated that β-endorphin may mitigate the toxicity of Aβ aggregation via direct molecular interactions, thereby contributing to an exercise-mediated reduction of AD risk. Combining biophysical characterizations in vitro with atomistic discrete molecular dynamics simulations in silico, a strong interaction is shown between β-endorphin and Aβ, where β-endorphins are located at the periphery to render a corona of their hetero-complexes with Aβ. Cell viability, immunofluorescence and western blotting assays further revealed that the corona shielded cellular exposure to Aβ aggregates and suppressed the toxicity of Aβ in vivo. This work offered a new molecular mechanism for the benefits of physical exercise, which may facilitate a rational design of future therapy and prevention strategies against AD and dementia.
Keywords: Alzheimer's disease; Aβ aggregation; corona; discrete molecular dynamics; β‐endorphin.
© 2025 The Author(s). Small published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Co-Aggregation of Syndecan-3 with β-Amyloid Aggravates Neuroinflammation and Cognitive Impairment in 5×FAD Mice.Int J Mol Sci. 2025 Jun 8;26(12):5502. doi: 10.3390/ijms26125502. Int J Mol Sci. 2025. PMID: 40564963 Free PMC article.
-
Computational Investigation of Coaggregation and Cross-Seeding between Aβ and hIAPP Underpinning the Cross-Talk in Alzheimer's Disease and Type 2 Diabetes.J Chem Inf Model. 2024 Jul 8;64(13):5303-5316. doi: 10.1021/acs.jcim.4c00859. Epub 2024 Jun 26. J Chem Inf Model. 2024. PMID: 38921060 Free PMC article.
-
Identification of isoAsp7-Aβ as a major Aβ variant in Alzheimer's disease, dementia with Lewy bodies and vascular dementia.Acta Neuropathol. 2024 Dec 3;148(1):78. doi: 10.1007/s00401-024-02824-9. Acta Neuropathol. 2024. PMID: 39625512 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
The Amyloid-Beta Clearance: From Molecular Targets to Glial and Neural Cells.Biomolecules. 2023 Feb 7;13(2):313. doi: 10.3390/biom13020313. Biomolecules. 2023. PMID: 36830682 Free PMC article.
References
-
- Nasica‐Labouze J., Nguyen P. H., Sterpone F., Berthoumieu O., Buchete N. V., Cote S., De Simone A., Doig A. J., Faller P., Garcia A., Laio A., Li M. S., Melchionna S., Mousseau N., Mu Y., Paravastu A., Pasquali S., Rosenman D. J., Strodel B., Tarus B., Viles J. H., Zhang T., Wang C., Derreumaux P., Chem. Rev. 2015, 115, 3518. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- P20 GM121342/GM/NIGMS NIH HHS/United States
- 172644KYSB20210011/Fundamental Research Funds for the Provincial Universities of Zhejiang
- 2024A1515011244/Natural Science Foundation of Guangdong Province
- 32171390/National Outstanding Youth Science Fund Project of National Natural Science Foundation of China
- 2022YFC2409700/Ministry of Science and Technology of China
- 24PJA015/Shanghai Magnolia Talent Plan Pujiang Project
- 2023A0505050123/Natural Science Foundation of Guangdong Province
- 11904189/National Outstanding Youth Science Fund Project of National Natural Science Foundation of China
- 2023B1515020104/Natural Science Foundation of Guangdong Province
- R35 GM145409/GM/NIGMS NIH HHS/United States
- 2024YFE0104500/Ministry of Science and Technology of China
- R35GM145409/GM/NIGMS NIH HHS/United States
- 2021YFA1200900/Ministry of Science and Technology of China
- P20GM121342/GM/NIGMS NIH HHS/United States
- 2023J078/Natural Science Foundation of Ningbo Municipality
- National Key Research and Development Program
- 32201154/National Outstanding Youth Science Fund Project of National Natural Science Foundation of China
- 82261138630/National Outstanding Youth Science Fund Project of National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical

