Immunoprofile of some surface and cytoplasmic peripheral cell adhesion molecules in oral squamous cell carcinoma
- PMID: 40384204
- PMCID: PMC12236281
- DOI: 10.47162/RJME.66.1.17
Immunoprofile of some surface and cytoplasmic peripheral cell adhesion molecules in oral squamous cell carcinoma
Abstract
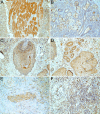
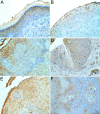
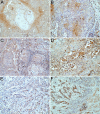
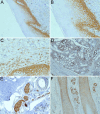
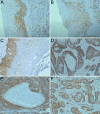
Despite the recent advances in diagnosis and treatment, oral squamous cell carcinoma (OSCC) continues to have a low overall survival rate (around 50%), being a tumor with high locoregional aggressiveness and high risk of lymph node (LN) dissemination. Such behavior can also be explained by the alteration of the expression of adhesion molecules, allowing tumor cells to invade surrounding tissues and make them capable of metastasizing. In this regard, we initiated a study on the immunohistochemical expression of Integrin alphavbeta6 (Integrin αvβ6), CD44 and Ezrin in OSCCs. A number of 39 such tumors with various locations in the oral cavity were investigated by enzymatic immunohistochemistry together with several samples of oral mucosa and oral dysplastic lesions. Using integrated optical density (IOD) as a method to quantify, we observed that the reactivity of these three markers decreased in the progression of dysplastic lesions and in the transition from well to moderately and poorly differentiated tumors. Also, in both conditions we noticed a shift of pattern reactivity from the continuous membrane to discontinuous membrane and cytoplasmic one, even to a nuclear pattern. In addition, the reactivity of the three markers was more evident in the invasion front and especially in these tumors with discohesive growth patterns. All this suggests the involvement of these adhesion molecules in the processes of transformation and malignant progression of OSCCs. It also explains their possible involvement in locoregional aggressiveness and LN dissemination.
Keywords: CD44; Ezrin; Integrin αvβ6; dysplastic lesions; immunohistochemistry; oral squamous cell carcinoma.
Conflict of interest statement
The authors declare that there is no conflict of interests regarding the publication of this paper. All authors read and approved the final manuscript.
Figures





Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
The Patterns of P53, E-Cadherin, β-Catenin, CXCR4 and Podoplanin Expression in Oral Squamous Cell Carcinoma Suggests a Hybrid Invasion Model: an Immunohistochemical Study on Tissue Microarrays.Head Neck Pathol. 2025 Jan 7;19(1):6. doi: 10.1007/s12105-024-01745-z. Head Neck Pathol. 2025. PMID: 39776043
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Bioinformatics identification and validation of m6A/m1A/m5C/m7G/ac4 C-modified genes in oral squamous cell carcinoma.BMC Cancer. 2025 Jul 1;25(1):1055. doi: 10.1186/s12885-025-14216-7. BMC Cancer. 2025. PMID: 40597017 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
-
- Moeckelmann N, Ebrahimi A, Tou YK, Gupta R, Low THH, Ashford B, Ch’ng S, Palme CE, Clark JR. Prognostic implications of the 8th edition American Joint Committee on Cancer (AJCC) Staging System in oral cavity squamous cell carcinoma. Oral Oncol. 2018;85:82–86. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

