Tailoring Spectral Response and First Hyperpolarizability of Aryl-Substituted BODIPY-Based 'Push-Pull' Chromophores: Influence of Medium and Structural Modifications
- PMID: 40384258
- PMCID: PMC12207575
- DOI: 10.1021/acs.jpca.5c00383
Tailoring Spectral Response and First Hyperpolarizability of Aryl-Substituted BODIPY-Based 'Push-Pull' Chromophores: Influence of Medium and Structural Modifications
Abstract
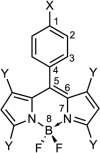
The medium plays a pivotal role in dictating the extent of intramolecular charge transfer (ICT) in a molecule, which could be useful in tuning its spectral and nonlinear optical (NLO) response properties. Tuning of ICT in a π-conjugated electronic donor-acceptor molecule has been utilized to modulate the absorption and emission maxima, as well as the first hyperpolarizability (β) of the so-called "push-pull" chromophores. Molecules with boron dipyrromethene (BODIPY)-based acceptors became popular in recent years for their unique photophysical properties, ease of synthesis, and high thermal stability. In this article, we present a quantum chemical investigation of the influence of the medium on the ICT process of some novel aryl-substituted BODIPY molecules. This influence ultimately modulates their absorption, emission, and nonlinear optical (NLO) properties. Both static and frequency-dependent β for the second harmonic generation are investigated along with the Pockels effect. Density functional theory (DFT) and time-dependent DFT (TDDFT) calculations using the long-range corrected CAM-B3LYP functional were employed in the present study. Restricting the rotation of the aryl ring through the incorporation of methyl groups to the BODIPY moiety enhances the fluorescence decay rate of the molecule. Both electronic and vibrational contributions to the static β are considered. A significant increase in β has been observed in polar solvents, compared to that in the gas phase. An interplay between structural and electronic effects was found to dictate the properties investigated. Our results shed light on the ICT process in the studied BODIPY dyes and could be useful in tuning their spectral properties as well as formulating design principles of novel NLO materials for future technological applications.
Figures


References
-
- Nonlinear optical properties of organic molecules and crystals V1, Chemla, D. S. , Ed.; Elsevier, 2012; Vol. 1.
-
- Computational aspects of electric polarizability calculations: Atoms, molecules and clusters, Maroulis, G. , Ed.; IOS press, 2006.
-
- Non-linear optical properties of matter, Papadopoulos, M. G. ; Sadlej, A. J. ; Leszczynski, J. , Eds.; Springer, 2006.
-
- Zyss J., Chemla D., Nicoud J.. Demonstration of efficient nonlinear optical crystals with vanishing molecular dipole moment: Second-harmonic generation in 3-methyl-4-nitropyridine-1-oxide. J. Chem. Phys. 1981;74:4800–4811. doi: 10.1063/1.441759. - DOI
-
- KARAKAŞ A., Koc Z. E., Fridrichova M., Němec P., Kroupa J.. The investigation of second-order nonlinear optical properties of p-nitrophenylazoaniline: second harmonic generation and ab initio computations. J. Theor. Comput. Chem. 2012;11:209–221. doi: 10.1142/S0219633612500149. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

