Pharmacodynamic Effect of Different Dosage Regimes of Oseltamivir in Severe Influenza Patients Requiring Mechanical Ventilation: A Multicentre Randomised Controlled Trial
- PMID: 40384328
- PMCID: PMC12086322
- DOI: 10.1111/irv.70109
Pharmacodynamic Effect of Different Dosage Regimes of Oseltamivir in Severe Influenza Patients Requiring Mechanical Ventilation: A Multicentre Randomised Controlled Trial
Abstract
Background and objectives: This randomised controlled trial evaluated whether higher doses of oseltamivir would improve virological and clinical outcomes in severe influenza patients requiring invasive mechanical ventilation.
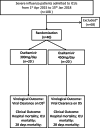
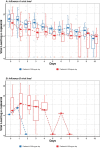
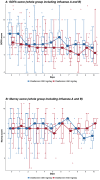
Methods: Forty intubated adult patients with severe influenza A or B from four intensive care units in Hong Kong were enrolled and randomised to receive either a double dose (300 mg/day) or a triple dose (450 mg/day) of oseltamivir for 10 days. Baseline data were collected, and outcomes were assessed daily using SOFA and Murray scores. Viral RNA was quantified from nasopharyngeal and tracheal aspirates. The primary outcome was the viral clearance rate after 5 days of treatment; secondary outcomes included 28-day and hospital mortality rates, changes in viral load, and serial SOFA and Murray scores.
Results: Viral clearance rates after 5 days of treatment were low and similar between the double (3/20, 15%) and triple-dose groups (2/20, 10%). No significant differences were observed in 28-day mortality, hospital mortality, ICU length of stay or duration of mechanical ventilation between the double and triple-dose groups. However, patients receiving triple doses exhibited a faster decline in influenza A viral load but had a longer hospital length of stay.
Conclusions: Triple doses of oseltamivir did not significantly improve virological or clinical outcomes compared with double doses in severe influenza.
Keywords: mechanical ventilation; oseltamivir; severe influenza.
© 2025 The Author(s). Influenza and Other Respiratory Viruses published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Effect of double dose oseltamivir on clinical and virological outcomes in children and adults admitted to hospital with severe influenza: double blind randomised controlled trial.BMJ. 2013 May 30;346:f3039. doi: 10.1136/bmj.f3039. BMJ. 2013. PMID: 23723457 Free PMC article. Clinical Trial.
-
A prospective intervention study on higher-dose oseltamivir treatment in adults hospitalized with influenza a and B infections.Clin Infect Dis. 2013 Dec;57(11):1511-9. doi: 10.1093/cid/cit597. Epub 2013 Sep 17. Clin Infect Dis. 2013. PMID: 24046309 Clinical Trial.
-
Neuraminidase inhibitor resistance after oseltamivir treatment of acute influenza A and B in children.Clin Infect Dis. 2009 Feb 15;48(4):389-96. doi: 10.1086/596311. Clin Infect Dis. 2009. PMID: 19133796
-
Oseltamivir in seasonal, pandemic, and avian influenza: a comprehensive review of 10-years clinical experience.Adv Ther. 2011 Nov;28(11):927-59. doi: 10.1007/s12325-011-0072-7. Epub 2011 Nov 1. Adv Ther. 2011. PMID: 22057727 Free PMC article. Review.
-
Oseltamivir: a review of its use in influenza.Drugs. 2001;61(2):263-83. doi: 10.2165/00003495-200161020-00011. Drugs. 2001. PMID: 11270942 Review.
References
-
- Muthuri S. G., Venkatesan S., Myles P. R., et al., “Effectiveness of Neuraminidase Inhibitors in Reducing Mortality in Patients Admitted to Hospital With Influenza a H1N1pdm09 Virus Infection: A Meta‐Analysis of Individual Participant Data,” Lancet Respiratory Medicine 2, no. 5 (2014): 395–404, 10.1016/S2213-2600(14)70041-4. - DOI - PMC - PubMed
-
- Govorkova E. A., Ilyushina N. A., Boltz D. A., Douglas A., Yilmaz N., and Webster R. G., “Efficacy of Oseltamivir Therapy in Ferrets Inoculated With Different Clades of H5N1 Influenza Virus,” Antimicrobial Agents and Chemotherapy 51, no. 4 (2007): 1414–1424, 10.1128/AAC.01312-06. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

