Associations Between Cortical Iron Accumulation and Memory in Patients With Amnestic Mild Cognitive Impairment and in Cognitively Normal Individuals
- PMID: 40384339
- PMCID: PMC12086325
- DOI: 10.1002/brb3.70521
Associations Between Cortical Iron Accumulation and Memory in Patients With Amnestic Mild Cognitive Impairment and in Cognitively Normal Individuals
Abstract
Background and purpose: Brain iron accumulation is recognized as a cause and therapeutic target in Alzheimer's disease (AD). We investigated the differences in both volume and iron accumulation between cognitively normal (CN) older adults and patients with amnestic mild cognitive impairment (aMCI). Additionally, we assessed which combination of these measures best explains the group differences in visual and verbal memory performance.
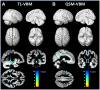
Materials and methods: We retrospectively analyzed data from 48 patients with aMCI and 33 age-matched CN individuals. Structural differences were investigated using voxel-based comparisons of T1-weighted magnetic resonance images. Differences in iron accumulation were investigated using voxel-based comparisons of quantitative susceptibility mapping (QSM) images. Subsequently, significant clusters from these voxel-based analyses (amygdala, posterior cingulate cortex, precuneus, lateral occipital cortex, and pericalcarine cortex) were entered into a stepwise regression to predict verbal and visual memory scores, while accounting for age, sex, and education as covariates.
Results: In comparison to CN, patients with aMCI had significantly lower scores in both verbal and visual memory tests (p < 0.001). The T1-weighted voxel-based morphometry (VBM) results showed significant hippocampal atrophy in the aMCI group relative to CN individuals. The QSM-VBM results showed increased iron accumulation in the amygdala, posterior cingulate cortex, precuneus, lateral occipital cortex, and pericalcarine cortex (FWE-corrected p < 0.05). Lower hippocampal volume (B = 2015.91, SE = 469.61, p < 0.001) and higher posterior cingulate cortex susceptibility (B = -189.63 SE = 89.11, p = 0.037) were significant predictors of verbal memory. For visual memory, higher lateral occipital susceptibility (B = -659. 96, SE = 253.03, p = 0.011) was significant imaging predictor.
Conclusions: These results suggest that iron accumulates in regions where atrophy has not yet occurred, suggesting that iron may serve as an earlier imaging marker of neurodegeneration compared to volume atrophy. Further studies are needed to investigate the longitudinal relationship between brain volume and iron accumulation during cognitive decline.
Keywords: iron; memory; mild cognitive impairment; quantitative susceptibility mapping.
© 2025 The Author(s). Brain and Behavior published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest related to the content of this article.
Figures

References
-
- Albert, M. S. , DeKosky S. T., Dickson D., et al. 2011. “The Diagnosis of Mild Cognitive Impairment due to Alzheimer's Disease: Recommendations from the National Institute on Aging‐Alzheimer's Association Workgroups on Diagnostic Guidelines for Alzheimer's Disease.” Alzheimer's & Dementia 7, no. 3: 270–279. 10.1016/j.jalz.2011.03.008. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

