BicD and MAP7 Collaborate to Activate Homodimeric Drosophila Kinesin-1 by Complementary Mechanisms
- PMID: 40384341
- PMCID: PMC12086504
- DOI: 10.1111/tra.70008
BicD and MAP7 Collaborate to Activate Homodimeric Drosophila Kinesin-1 by Complementary Mechanisms
Abstract
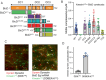
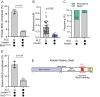
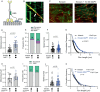
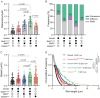
The folded auto-inhibited state of kinesin-1 is stabilized by multiple weak interactions and binds poorly to microtubules. Here we investigate the extent to which homodimeric Drosophila kinesin-1 lacking light chains is activated by the dynein activating adaptor Drosophila BicD. We show that one or two kinesins can bind to the central region of BicD (CC2), a region distinct from that which binds dynein-dynactin (CC1) and cargo-adaptor proteins (CC3). Kinesin light chain significantly reduces the amount of kinesin bound to BicD and thus regulates this interaction. Binding of BicD to kinesin enhances processive motion, suggesting that the adaptor relieves kinesin auto-inhibition. In contrast, the kinesin-binding domain of microtubule-associated protein 7 (MAP7) has minimal impact on the fraction of motors moving processively while full-length MAP7 enhances kinesin-1 recruitment to the microtubule and run length because of its microtubule-binding domain. BicD thus relieves auto-inhibition of kinesin, while MAP7 enhances motor engagement with the microtubules. When BicD and MAP7 are combined, the most robust activation of kinesin-1 occurs, highlighting the crosstalk between adaptors and microtubule-associated proteins in regulating transport.
Keywords: adaptor protein; dynein; kinesin; microtubule; processivity.
© 2025 The Author(s). Traffic published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Update of
-
BicD and MAP7 collaborate to activate homodimeric Drosophila kinesin-1 by complementary mechanisms.bioRxiv [Preprint]. 2025 Jan 14:2025.01.11.632512. doi: 10.1101/2025.01.11.632512. bioRxiv. 2025. Update in: Traffic. 2025 Apr-Jun;26(4-6):e70008. doi: 10.1111/tra.70008. PMID: 39868150 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

