Alpha-Synuclein as a Potential Biomarker for Inclusion Body Myositis in Blood and Muscle
- PMID: 40384347
- PMCID: PMC12086613
- DOI: 10.1111/nan.70019
Alpha-Synuclein as a Potential Biomarker for Inclusion Body Myositis in Blood and Muscle
Abstract
Aims: Diagnosis of inclusion body myositis (IBM) is difficult and currently based on a combination of clinical and (immuno)histological findings. Biomarkers facilitating the diagnostic process are needed. Alpha-synuclein (αSN) aggregates are a known histological feature of IBM, but there is a lack of information on their diagnostic relevance. Furthermore, serum αSN concentrations in IBM have not been investigated.
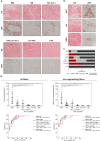
Methods: Immunohistochemical staining for αSN was performed on 63 biopsies (19 IBM, 21 other inflammatory myopathies, 20 other myopathies and 3 healthy controls), and αSN reactive fibres were quantified. The serum concentration of αSN was determined by ELISA in 156 serum samples (11 IBM, 25 other inflammatory myopathies, 53 hereditary myopathies, 30 mitochondriopathies and 37 healthy controls).
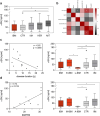
Results: The proportion of fibres with αSN immunoreactivity was significantly higher in IBM compared to all groups (p < 0.001) and discriminated IBM against all other neuromuscular disorders with a sensitivity of 79% and a specificity of 85%, which further improved when only non-regenerating fibres were examined. In serum, αSN concentrations in IBM were generally not different from healthy controls. However, serum concentrations were inversely correlated with disease duration (r = -0.62, p = 0.04) and positively correlated with the IBM functional rating scale (r = 0.74, p = 0.01). Consequently, stratification according to these clinical parameters showed significantly lower serum αSN concentrations in late-stage, more severely affected patients.
Conclusions: αSN reactivity may serve as an additional immunohistochemical marker for IBM diagnosis. Furthermore, this study indicates that αSN serum concentrations decrease with disease duration and clinical deterioration. Therefore, serum αSN may be provisionally considered a monitoring biomarker in IBM, pending further studies.
Keywords: IBM; biomarker; inclusion body; myositis; α‐synuclein.
© 2025 The Author(s). Neuropathology and Applied Neurobiology published by John Wiley & Sons Ltd on behalf of British Neuropathological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

