Clonal Hematopoiesis and Solid Cancers
- PMID: 40384356
- PMCID: PMC12317386
- DOI: 10.1111/cas.70097
Clonal Hematopoiesis and Solid Cancers
Abstract
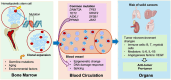
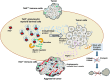
Clonal hematopoiesis refers to the expansion of hematopoietic stem cells harboring somatic mutations, a phenomenon increasingly recognized in aging populations. This review highlights the emerging relationship between clonal hematopoiesis and solid cancers, focusing on the prevalence and impact of clonal hematopoiesis-associated mutations such as DNMT3A, TET2, ASXL1, and TP53 in tumorigenesis. Key risk factors for the co-occurrence of clonal hematopoiesis and solid cancers, including germline genetic factors, aging, and environmental factors, are also discussed. We explore how clonal hematopoiesis mutations shape the tumor microenvironments in solid cancers by modulating immunoregulation, inflammation, and angiogenesis, thereby contributing to tumor progression. These findings underscore the dual role of clonal hematopoiesis as both a marker of cancer risk and a potential driver of solid cancer progression. The clinical implications of clonal hematopoiesis are also considered, including the prognostic value, impact on treatment response, and potential as a therapeutic target. Future directions are outlined to advance our understanding of clonal hematopoiesis and to exploit its clinical potential for cancer management.
Keywords: TET2 mutations; T‐cell lymphomas; aging; clonal hematopoiesis; solid cancers; tumor microenvironments.
© 2025 The Author(s). Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare no conflicts of interest. Dr. Ishikawa, Shumpei and Dr. Sakata‐Yanagimoto, Mamiko, are editorial board members of Cancer Science.
Figures
References
-
- Fey M. F., Liechti‐Gallati S., von Rohr A., et al., “Clonality and X‐Inactivation Patterns in Hematopoietic Cell Populations Detected by the Highly Informative M27 Beta DNA Probe,” Blood 83, no. 4 (1994): 931–938. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous