Genomic context analysis enables the discovery of an unusual NAD-dependent racemase in phosphonate catabolism
- PMID: 40384479
- PMCID: PMC12366243
- DOI: 10.1111/febs.70130
Genomic context analysis enables the discovery of an unusual NAD-dependent racemase in phosphonate catabolism
Abstract
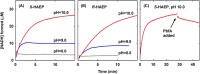
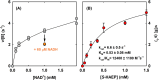
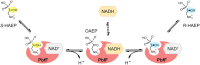
Phosphonates are organic molecules containing a direct carbon-phosphorus (C-P) bond. They are chemically sturdy compounds that can, however, be degraded by environmental microorganisms. In the frame of bacterial phosphonate catabolism, we recently reported the discovery of (R)-1-hydroxy-2-aminoethylphosphonate ammonia-lyase (PbfA), a lyase acting on the natural compound (R)-2-amino-1-hydroxyethylphosphonate (R-HAEP). PbfA converts R-HAEP into phosphonoacetaldehyde (PAA), which can be subsequently processed and cleaved by further enzymes. However, PbfA is not active toward S-HAEP (the enantiomer of R-HAEP), whose metabolic fate remained unknown. We now describe the identification of a racemase, discovered through genomic context analysis, which converts S-HAEP into R-HAEP, thereby enabling degradation of S-HAEP. We propose for this enzyme the official name 2-amino-1-hydroxyethylphosphonate racemase (shorthand PbfF). To our knowledge, PbfF is the first NAD-dependent racemase ever described and is structurally unrelated to other known NAD-dependent isomerases. The enzyme uses NAD+ as a cofactor, is inhibited by NADH, and shows catalytic parameters comparable to those of other racemases acting on similar substrates. The presence of a pathway for the breakdown of S-HAEP in numerous bacteria suggests that this compound may be more common in the environment than currently appreciated. Notably, the route for S-HAEP degradation appears to have developed through a mechanism of retrograde metabolic evolution.
Keywords: 2‐amino‐1‐hydroxyethylphosphonate; Alphaproteobacteria; NAD‐dependent isomerase; phosphonate degradation; racemase.
© 2025 The Author(s). The FEBS Journal published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Horsman GP & Zechel DL (2017) Phosphonate biochemistry. Chem Rev 117, 5704–5783. - PubMed
-
- Hilderbrand RL & Henderson T (1983) Phosphonic acids in nature. In The Role of Phosphonates in Living Systems (Hilderbrand RL, ed.), pp. 5–28. CRC Press, Boca Raton, FL, USA.
-
- Kafarski P (2019) Phosphonates: their natural occurrence and physiological role. In Contemporary Topics about Phosphorus in Biology and Materials (Churchill DG, Sikirić MD, Čolović B & Milhofer HF, eds), pp. 1–19. Intech Open, London, UK.
-
- Rott E, Steinmetz H & Metzger JW (2018) Organophosphonates: a review on environmental relevance, biodegradability and removal in wastewater treatment plants. Sci Total Environ 615, 1176–1191. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

