Quantitative assessment of biomechanical changes in oral lesions at different cancerous stages using optical coherence elastography
- PMID: 40384691
- PMCID: PMC12082576
- DOI: 10.21037/qims-24-2359
Quantitative assessment of biomechanical changes in oral lesions at different cancerous stages using optical coherence elastography
Abstract
Background: Oral cancer is the sixth most common cancer worldwide. The detection, prevention, and control of oral potentially malignant disorders (OPMDs) at early stages is imperative to reduce the incidence of oral cancer. This study analyzed ultrastructural and biomechanical tissue properties during tongue cancer development in Sprague-Dawley (SD) rats using optical coherence elastography (OCE). Our investigation examined the changes associated with oral cancer pathogenesis and explored the feasibility of OCE as an early diagnostic tool for oral cancer.
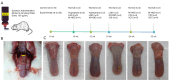
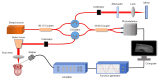
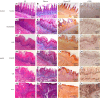
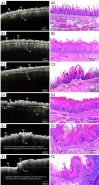
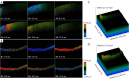
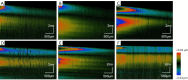
Methods: In this study, 4-nitroquinoline-1-oxide (4NQO) was used to induce oral carcinogenesis in SD rats. In total, 10 normal tissues, five hyperplastic lesions, eight low-grade dysplasias (LGDs), eight moderate-high-grade dysplasias (M-HGDs), seven carcinomas in situ (CISs), and seven squamous cell carcinomas (SCCs) were examined. The oral stroma changes were sequentially imaged by in vivo shaker-based OCE (shaker-OCE). The changes in the oral stroma from normal to hyperplasia, atypical hyperplasia, CIS, and cancer were determined using OCE, and histological findings such as extracellular matrix (ECM) components (including collagen and elastic fibers) and the expression of cancer-associated fibroblasts (CAFs) were compared at different stages of tongue cancer development.
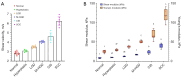
Results: The findings showed that OCE imaging could be used to accurately distinguish between normal, hyperplasia, atypical hyperplasia, CIS, and oral cancer. Additionally, there were significant differences in the tongue tissue biomechanics across the different lesion levels (P<0.05). Further, as the malignancy of the tongue cancer progressed in the SD rats, the level of collagen fibers gradually increased, showing a positive correlation (r=0.353, P<0.05), while the level of elastic fiber expression gradually decreased, showing a negative correlation (r=-0.776, P<0.05). The alpha-smooth muscle actin (α-SMA) scores of CIS and SCC were statistically significantly higher than those of normal, simple hyperplasia, mild atypical hyperplasia, and moderate-high atypical hyperplasia (P<0.05).
Conclusions: The ability of the shaker-OCE system to obtain the structural and biomechanical characteristics of tongue tissues in a non-invasive, real-time manner was confirmed by this study. It also showed notable benefits in terms of early diagnosis and the dynamic monitoring of tongue cancer. The systematic validation of the physiopathological model revealed a strong correlation between the elastic properties of cancerous tissues and pathological evolution, which provides a theoretical basis and experimental evidence for the clinical application of OCE technology.
Keywords: Oral cancer; dysplasia; early diagnosis; elasticity imaging techniques; optical coherence elastography (OCE).
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2359/coif). The authors have no conflicts of interest to declare.
Figures

References
-
- Chen PH, Wu CH, Chen YF, Yeh YC, Lin BH, Chang KW, Lai PY, Hou MC, Lu CL, Kuo WC. Combination of structural and vascular optical coherence tomography for differentiating oral lesions of mice in different carcinogenesis stages. Biomed Opt Express 2018;9:1461-76. 10.1364/BOE.9.001461 - DOI - PMC - PubMed
-
- Romano A, Di Stasio D, Petruzzi M, Fiori F, Lajolo C, Santarelli A, Lucchese A, Serpico R, Contaldo M. Noninvasive Imaging Methods to Improve the Diagnosis of Oral Carcinoma and Its Precursors: State of the Art and Proposal of a Three-Step Diagnostic Process. Cancers (Basel) 2021;13:2864. 10.3390/cancers13122864 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
