Embedding a commitment to equitable global access into basic and early-phase translational research
- PMID: 40384749
- PMCID: PMC12083207
- DOI: 10.1017/cts.2024.691
Embedding a commitment to equitable global access into basic and early-phase translational research
Abstract
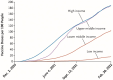
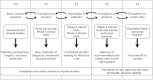
The COVID-19 pandemic laid bare the inequities in U.S. healthcare in ways that captured public attention and reinforced the need to view all of healthcare through an equity lens. It also exposed global inequities in access to healthcare technologies. At Rockefeller University, we participate in the entire spectrum of translational research, but our focus is in the areas of basic research and new methods to prevent, diagnose, and treat disease, extending to proof of concept preclinical and Phase 1 studies. Since we believe that all phases of translational research should have an equity lens, we have instituted an initiative to encourage thought and planning about global equitable access to discoveries made by our trainee Clinical Scholars and faculty, even at the earliest phases of basic research. Assuring global equitable access to new technologies requires addressing at least 3 different aspects of new technology: 1. Patenting and licensing, 2. Manufacturing, and 3. Dissemination and implementation in low- and middle-income countries. In this review, I focus on patenting and licensing and offer ten questions for inventors to consider in discussing licensing their technologies with technology transfer officers to maximize equitable global access to the technologies they create.
Keywords: Technology transfer; health equity; license; manufacturing; patent.
© The Author(s) 2025.
Conflict of interest statement
B.S.C. declares no competing interests for the material in this review.
Figures
Similar articles
-
University patenting and licensing practices in the United Kingdom during the first year of the COVID-19 pandemic.Glob Public Health. 2022 May;17(5):641-651. doi: 10.1080/17441692.2022.2049842. Epub 2022 Mar 17. Glob Public Health. 2022. PMID: 35298347
-
The Future of Epidemic and Pandemic Vaccines to Serve Global Public Health Needs.Vaccines (Basel). 2023 Mar 17;11(3):690. doi: 10.3390/vaccines11030690. Vaccines (Basel). 2023. PMID: 36992275 Free PMC article. Review.
-
Global health equity in United Kingdom university research: a landscape of current policies and practices.Health Res Policy Syst. 2016 Oct 10;14(1):76. doi: 10.1186/s12961-016-0148-6. Health Res Policy Syst. 2016. PMID: 27724907 Free PMC article.
-
Enabling equitable and affordable access to novel therapeutics for pandemic preparedness and response via creative intellectual property agreements.Wellcome Open Res. 2024 Jul 15;9:374. doi: 10.12688/wellcomeopenres.22645.1. eCollection 2024. Wellcome Open Res. 2024. PMID: 39184131 Free PMC article.
-
COVID-19 Vaccination and Public Health: Addressing Global, Regional, and Within-Country Inequalities.Vaccines (Basel). 2024 Aug 4;12(8):885. doi: 10.3390/vaccines12080885. Vaccines (Basel). 2024. PMID: 39204011 Free PMC article. Review.
References
-
- Guebert JM, Bubela T. Implementing socially responsible licensing for global health: beyond neglected diseases. Sci Transl Med. 2014;6:260cm211. - PubMed
-
- Hunter DJ, Abdool Karim SS, Baden LR, et al. Addressing vaccine inequity - covid-19 vaccines as a global public good. N Engl J Med. 2022;386:1176–1179. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
