Multikinase inhibition-mediated proliferative vitreoretinopathy therapy by nanoparticles in rabbits
- PMID: 40384768
- PMCID: PMC12085219
Multikinase inhibition-mediated proliferative vitreoretinopathy therapy by nanoparticles in rabbits
Abstract
Purpose: To investigate the efficacy of nanoparticles in treating proliferative vitreoretinopathy (PVR) through clinical observation, histology, and immunohistochemistry, despite unsatisfactory surgical outcomes and failed therapies for the current PVR treatment.
Design: Twelve rabbits were divided into control and nintedanib (NTB) groups. The rabbits underwent weekly ophthalmologic examinations over a period of four weeks.
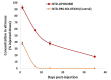
Methods: At the end of the fourth week, the rabbits' eyes were removed for histological and immunohistochemical evaluation. Three additional rabbits outside the PVR model were administered a 0.5% NTB-loaded liposomal formulation in one eye. The drug concentrations in the vitreous samples were determined using high-pressure liquid chromatography on days 1, 7, 14, and 35.
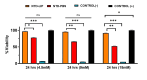
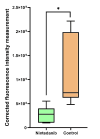
Results: The PVR stages were low in the NTB group, and there was no significant difference between the NTB and control groups (p = 0.108). However, it is worth noting that the group treated with NTB had significantly fewer epiretinal membrane formations during the histological evaluation. In addition, the corrected fluorescence intensity measurement of the subjects for collagen-1 in the NTB group was significantly lower than that in the control group (p = 0.004). Most importantly, no significant adverse effects were observed.
Conclusions: Our study has provided preclinical support for a liposomal formulation containing NTB that, with single-dose administration, has the potential to be effective in vivo in preventing the development of PVR and its correlated pathologies without causing any significant side effects.
Copyright © 2025 Molecular Vision.
Figures










References
-
- Hilton G, Machemer R, Michels R, Okun E, Schepens C, Schwartz A. The classification of retinal detachment with proliferative vitreoretinopathy. Ophthalmology. 1983;90:121–5. - PubMed
-
- Ryan SJ. The pathophysiology of proliferative vitreoretinopathy in its management. Am J Ophthalmol. 1985;100:188–93. - PubMed
-
- Pastor JC. Proliferative vitreoretinopathy: an overview. Surv Ophthalmol. 1998;43:3–18. - PubMed
-
- Pastor JC, de la Rúa ER, Martín F. Proliferative vitreoretinopathy: risk factors and pathobiology. Prog Retin Eye Res. 2002;21:127–44. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

