Pharmacological Activation of AMP-activated Protein Kinase Ameliorates Liver Fibrosis in a Metabolic Dysfunction-Associated Steatohepatitis Mouse Model
- PMID: 40384865
- PMCID: PMC12080390
- DOI: 10.7150/ijbs.108731
Pharmacological Activation of AMP-activated Protein Kinase Ameliorates Liver Fibrosis in a Metabolic Dysfunction-Associated Steatohepatitis Mouse Model
Abstract
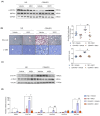
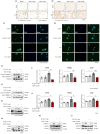
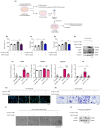
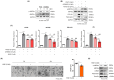
Metabolic dysfunction-associated steatohepatitis (MASH) is a significant contributor to hepatocellular carcinoma (HCC). To validate AMPK activation as a therapeutic strategy for MASH-associated liver fibrosis, we investigated the effects of a 4-chloro-benzenesulfonamide derivative named KN21, a novel AMPK activator, on the liver fibrogenic process in a MASH model. In mice fed a choline-deficient, L-amino acid-defined, high fat diet (CDAHFD), KN21 reduced hepatic steatosis, lipid accumulation, and liver fibrosis. In hepatocyte cells treated with palmitic acid and oleic acid (PO), KN21 attenuated lipid accumulation and the release of reactive oxygen species (ROS) and fibrotic mediators. Hepatic stellate cells stimulated with hepatocyte-derived conditioned medium (CM) exhibited increased expression of fibrosis markers, whereas hepatic stellate cells exposed to CM from KN21-treated hepatocytes showed a decrease of fibrosis marker expression. Additionally, KN21 inhibited the activation of human hepatic stellate cells and demonstrated potent antifibrotic activity. These findings underscore the therapeutic potential of pharmacological AMPK activation for the treatment of MASH-associated liver fibrosis.
Keywords: 4-chloro-benzenesulfonamide derivative; AMP-activated protein kinase (AMPK); lipid accumulation; liver fibrosis; metabolic dysfunction-associated steatohepatitis (MASH); synthetic AMPK activator.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
Natural molecule isoliquiritigenin mitigates MASH and liver fibrosis in mice by promoting autophagy through the PI3K/Akt signaling pathway.J Nutr Biochem. 2025 Feb;136:109808. doi: 10.1016/j.jnutbio.2024.109808. Epub 2024 Nov 19. J Nutr Biochem. 2025. PMID: 39571827
-
Fluorofenidone attenuates choline-deficient, l-amino acid-defined, high-fat diet-induced metabolic dysfunction-associated steatohepatitis in mice.Sci Rep. 2025 Mar 21;15(1):9863. doi: 10.1038/s41598-025-94401-7. Sci Rep. 2025. PMID: 40118958 Free PMC article.
-
Ebastine-mediated destabilization of E3 ligase MKRN1 protects against metabolic dysfunction-associated steatohepatitis.Cell Mol Life Sci. 2025 Jan 31;82(1):66. doi: 10.1007/s00018-024-05535-2. Cell Mol Life Sci. 2025. PMID: 39888429 Free PMC article.
-
Polyoxometalates Ameliorate Metabolic Dysfunction-Associated Steatotic Liver Disease by Activating the AMPK Signaling Pathway.Int J Nanomedicine. 2024 Oct 25;19:10839-10856. doi: 10.2147/IJN.S485084. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39479173 Free PMC article.
-
1-phenyl-3-methyl-5-pyrazolone activates the AMPK pathway to alleviate western-diet induced metabolic dysfunction-associated steatohepatitis in mice.Clin Nutr. 2025 Feb;45:136-147. doi: 10.1016/j.clnu.2024.12.033. Epub 2025 Jan 4. Clin Nutr. 2025. PMID: 39799716
References
-
- Dixon JB, Bhathal PS, O'brien PE. Nonalcoholic fatty liver disease: Predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. - PubMed
-
- Tu T, Calabro SR, Lee A, Maczurek AE, Budzinska MA, Warner FJ. et al. Hepatocytes in liver injury: Victim, bystander, or accomplice in progressive fibrosis? Journal of gastroenterology and hepatology. 2015;30:1696–704. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

