Epigenetic modifications in cardiac fibrosis: recent evidence of new pharmacological targets
- PMID: 40384946
- PMCID: PMC12081422
- DOI: 10.3389/fmolb.2025.1583446
Epigenetic modifications in cardiac fibrosis: recent evidence of new pharmacological targets
Abstract
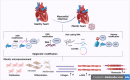
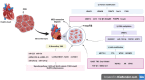
Cardiac fibrosis (CF) is characterized by the excessive deposition of collagen types I (COI I) and III (COI III), primarily mediated by cardiac fibroblasts (CFB). Recent advances in epigenetic research have enhanced our understanding of the molecular mechanisms underlying CF and have facilitated the identification of novel therapeutic strategies targeting key proteins and signaling pathways involved in its progression. Epigenetic modifications, including DNA methylation, histone modifications, and non-coding RNAs (ncRNAs), are structural and chemical alterations that regulate gene expression and cellular responses without changing the DNA sequence. Investigating the role of epigenetic enzymes in CF may reveal promising pharmacological targets. This review summarizes current evidence on epigenetic modifications implicated in CF and discusses their potential as therapeutic targets for modulating this pathological process.
Keywords: DNA methylation; cardiac fibroblasts; cardiac fibrosis; cardiovascular epigenetics; epigenetic modifications; non-coding RNA.
Copyright © 2025 Pérez, Gómez, Castellar-López, Araos, Mendoza-Torres and Bolívar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Non-coding RNAs as direct and indirect modulators of epigenetic mechanism regulation of cardiac fibrosis.Expert Opin Ther Targets. 2015 May;19(5):707-16. doi: 10.1517/14728222.2014.1001740. Epub 2015 Feb 4. Expert Opin Ther Targets. 2015. PMID: 25652534 Review.
-
Epigenomics in stress tolerance of plants under the climate change.Mol Biol Rep. 2023 Jul;50(7):6201-6216. doi: 10.1007/s11033-023-08539-6. Epub 2023 Jun 9. Mol Biol Rep. 2023. PMID: 37294468 Review.
-
Fibroblast Diversity and Epigenetic Regulation in Cardiac Fibrosis.Int J Mol Sci. 2024 May 30;25(11):6004. doi: 10.3390/ijms25116004. Int J Mol Sci. 2024. PMID: 38892192 Free PMC article. Review.
-
Roles of Epigenetics in Cardiac Fibroblast Activation and Fibrosis.Cells. 2022 Jul 30;11(15):2347. doi: 10.3390/cells11152347. Cells. 2022. PMID: 35954191 Free PMC article. Review.
-
Non-coding RNA-mediated epigenetic regulation of liver fibrosis.Metabolism. 2015 Nov;64(11):1386-94. doi: 10.1016/j.metabol.2015.08.004. Epub 2015 Aug 14. Metabolism. 2015. PMID: 26362725 Review.
References
-
- Cao Y., Lu L., Liu M., Li X. C., Sun R. R., Zheng Y., et al. (2014). Impact of epigenetics in the management of cardiovascular disease: a review. Eur. Rev. Med. Pharmacol. Sci. 18 (20), 3097–3104. - PubMed
-
- Chen C. J., Huang J. Y., Huang J. Q., Deng J. Y., Shangguan X. H., Chen A. Z., et al. (2023). Metformin attenuates multiple myeloma cell proliferation and encourages apoptosis by suppressing METTL3-mediated m6A methylation of THRAP3, RBM25, and USP4. Cell. Cycle 22, 986–1004. 10.1080/15384101.2023.2170521 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

