Mycobacterium tuberculosis modulates NUDT21-mediated alternative polyadenylation to enhance FTH1 expression in macrophages and promotes intracellular growth
- PMID: 40384984
- PMCID: PMC12081465
- DOI: 10.3389/fcimb.2025.1578163
Mycobacterium tuberculosis modulates NUDT21-mediated alternative polyadenylation to enhance FTH1 expression in macrophages and promotes intracellular growth
Abstract
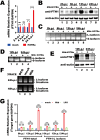
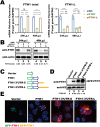
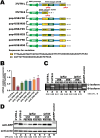
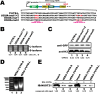
Ferritin heavy chain 1 (FTH1) is a key iron-storage protein that regulates iron availability, supports immune defense, and prevents iron-induced toxicity. During Mycobacterium tuberculosis (Mtb) infection, macrophages enhance FTH1 expression to sequestrate iron and limit Mtb growth. However, Mtb can exploit the host ferritinophagy pathway to degrade FTH1 and release iron, thereby promoting its survival. Although FTH1 plays an essential role in host-pathogen interaction during Mtb infection, its regulation remains unclear. Previous studies suggest that post-transcriptional mechanism, particularly alternative polyadenylation (APA), are critical in immune responses. We propose that APA, which determines the length of a transcript's 3'UTR, may regulate FTH1 expression during Mtb infection. Our study demonstrates that Mtb induces APA of FTH1 in macrophages, favoring the production of longer isoforms that enhance protein synthesis. Mechanistically, Mtb disrupts the interaction between NUDT21 and CPSF6, impairing NUDT21's ability to bind UGUA motifs in the FTH1 3'UTR, a key step in polyadenylation site selection. Silencing NUDT21 reduces macrophage bactericidal activity against Mtb, highlighting its role in immune defense. These findings reveal a novel Mtb-driven mechanism that enhances FTH1 expression via the NUDT21-mediated APA pathway in macrophages, suggesting that Mtb manipulates this process to promote its survival. This study provides new insights into tuberculosis pathogenesis and points to potential avenues for therapeutic exploration.
Keywords: FTH1; Mycobacterium tuberculosis; NUDT21; alternative polyadenylation; macrophage.
Copyright © 2025 Liu, Cai, Dai, Chen and Zeng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Alternative polyadenylation landscape of longissimus dorsi muscle with high and low intramuscular fat content in cattle.J Anim Sci. 2024 Jan 3;102:skae357. doi: 10.1093/jas/skae357. J Anim Sci. 2024. PMID: 39565284
-
IGF2BP2 binding to CPSF6 facilitates m6A-mediated alternative polyadenylation of PUM2 and promotes malignant progression in ovarian cancer.Clin Transl Med. 2025 Jul;15(7):e70388. doi: 10.1002/ctm2.70388. Clin Transl Med. 2025. PMID: 40629911 Free PMC article.
-
TRIM65 deficiency alleviates renal fibrosis through NUDT21-mediated alternative polyadenylation.Cell Death Differ. 2024 Nov;31(11):1422-1438. doi: 10.1038/s41418-024-01336-z. Epub 2024 Jun 29. Cell Death Differ. 2024. PMID: 38951701
-
Immune defense and immune evasion in Mycobacterium tuberculosis Infection: Inspirations and challenges for host directed therapy.Microb Pathog. 2025 Oct;207:107914. doi: 10.1016/j.micpath.2025.107914. Epub 2025 Jul 16. Microb Pathog. 2025. PMID: 40681004 Review.
-
Iron, Copper, and Zinc Homeostasis in the Battle between Macrophage and Mycobacterium Tuberculosis.Curr Med Chem. 2025;32(16):3155-3168. doi: 10.2174/0109298673283407231225140659. Curr Med Chem. 2025. PMID: 38231073 Review.
References
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

